Research Article Open Access
Sleeve Gastrectomy in Canine: Effect on Weight Loss, Inflammation and Oxidative Stress Markers while Leaving Blind Intestinal Loop in Place
Al-Wadani H, Marrar AN, Odeh AM and Safaya S*Department of Surgery and Al-Omran Scientific Chair, College of Medicine, King Faisal University, Al-hasa, Kingdom of Saudi Arabia
- *Corresponding Author:
- Surinder Safaya
Department of Surgery and Al-Omran Scientific Chair, College of Medicine
King Faisal University, Al-hasa, Kingdom of Saudi Arabia
Tel: 966562415573
E-mail: s_safaya@hotmail.com
Received date: May 03, 2017; Accepted date: May 15, 2017; Published date: May 17, 2017
Citation: Al-Wadani H, Marrar AN, Odeh AM, Safaya S (2017) Sleeve Gastrectomy in Canine: Effect on Weight Loss, Inflammation and Oxidative Stress Markers while Leaving Blind Intestinal Loop in Place. J Obes Weight Loss Ther 7:337. doi:10.4172/2165-7904.1000337
Copyright: © 2017 Al-Wadani H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
The aim of this study was to modify the current Sleeve gastrectomy surgical procedure by leaving behind the blind intestinal loop, with the purpose to overcome the complications of gastric bypass. Accordingly, we evaluated in Canine, the effectiveness of this approach, on the weight loss and markers of inflammation and oxidative stress. Our results suggest that this type of bypass surgery in Canine resulted in significant weight loss that was maintained up to six months. There was no evidence of post-operative leaks, infection or organ adhesion. However, oxidative stress and inflammation markers were elevated in the post-operative stomach but not in the nonfunctional blind intestinal loop, six months after surgery. Elevation of these markers, post surgically in the target organ suggesting that it may take longer time for these markers to come back to healthy pre-operative levels. The purpose of leaving behind the blind intestinal loop may allow reversing the bypass in future date due to any unintended complications such as excessive weight loss.
Keywords
Sleeve gastrectomy; Weight loss; Stress
Introduction
Sleeve gastrectomy (SG) involving gastric reservoir construction that preserves the atrium and pylorus with vagal innervation, followed by biliopancreatic diversion (BPD) with duodenal switch (DS) in the same session, in patients with acceptable operative risk. With the preservation of pylorus, there virtually are no restrictions on what patients can eat as compared to gastric bypass [1,2]. This two-step approach has been reported to decrease in peri-operative morbidity and mortality compared to one stage BPD [3].
Despite the perceived advantages of SG, this procedure is not widely performed. A recent study has compared SG with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB) and concluded that SG has better weight loss and fewer complications and thus may be a reasonable option for treatment of morbid obesity [4]. Long-term data on sleeve gastrectomy is not available; however recent studies are suggestive that bariatric surgery reduces the progression and mortality of no-insulin dependent diabetes [5].
Gastrointestinal hormone secretion changes after gastric bypass surgery, that bypasses duodenum and upper jejunum, would favor improvement of type 2 diabetes. Although gastric bypass produces rapid weight loss, is invariably associated with malabsorption of essential nutrients. It has been reported that in some patients undergone SG, feel less hungry as compared to gastric bypass, presumably because large portion of stomach that secretes hormones is removed [1,6]. Weight loss after bariatric surgery appears to a major contributor of diabetic improvement [7].
Weight loss induced by bariatric surgery is a major factor of diabetes improvement. However, in several studies, the resolution of diabetes has often been observed before a significant weight loss has been obtained. The early post-surgical improvement of diabetes suggested a major physio pathological role for changes in gut hormone secretion [8-11]. As a matter of fact, a decrease in plasma levels of ghrelin, an orexigenic peptide, has been described following gastric bypass for morbid obesity [12]. The involvement of other intestinal peptides like GLP-1 and neuropeptide YY or a decreased secretion of anti-incretin hormones has been proposed to explain the rapid remission of diabetes after bariatric surgery [13-17]. Roux-en-Y bypass (RYGB) that excludes the duodenum from the nutriments route modifies the gut microbial metabolic environment and that has been shown to improve insulin resistance more rapidly than sleeve gastrectomy [18].
Obesity has been shown to be associated with elevated inflammatory markers and heightened oxidative stress. Elevated cytokine production by gut mucosa has been shown in cases of Crohn’s disease, ulcerative colitis and other intestinal inflammatory conditions [19]. However visceral adipose tissue cytokines and adipokines are elevated during morbid obesity, insulin resistance and non-alcoholic fatty liver disease [20-22]. Gastric cytokine production in patients after SG has not been reported. Most of the reports on changes in inflammatory biomarkers after gastric bypass surgery have been reported for circulating plasma. From these studies it is apparent that there is reduction in the pro-inflammatory biomarker and increase in anti-inflammatory markers, independent of the degree of weight loss in the circulating plasma after RYGB [23].
Obese subjects undergoing RYGB preoperatively have been reported to have higher oxidative stress and inflammation as assessed by circulating plasma levels. However one year after surgery there was marked improvement in antioxidant protection and substantial decline in the oxidative stress and inflammatory markers [24-26].
In the present canine study, we modified the current SG bypass surgical procedure to overcome the complications of the sleeve gastrectomy and gastric bypass by leaving behind the blind intestinal loop. We determined the level oxidative stress and inflammatory markers in the pre and post-surgical tissue of the stomach and intestinal blind loop by biochemical and Immunocytochemical assays.
Materials and Methods
The study was approved by the institutional animal review board. Canine (six male and four female) used in the present study, were maintained on high protein and carbohydrate diet and weighed 25 kg on average. On the day of surgery, dogs were pre-medicated with intramuscular injections of atropine (0.2-0.4 mg/kg) and ketamine (15-20 mg/kg). Endotracheal intubation was followed by an intravenous line with lactated Ringer's solution and pentobarbital (20-30 mg/kg) to induce anesthesia and maintained with isoflurane (1.5%) and oxygen. The animals were kept in supine position and an orogastric tube was inserted for evacuation of stomach.
Surgical procedure
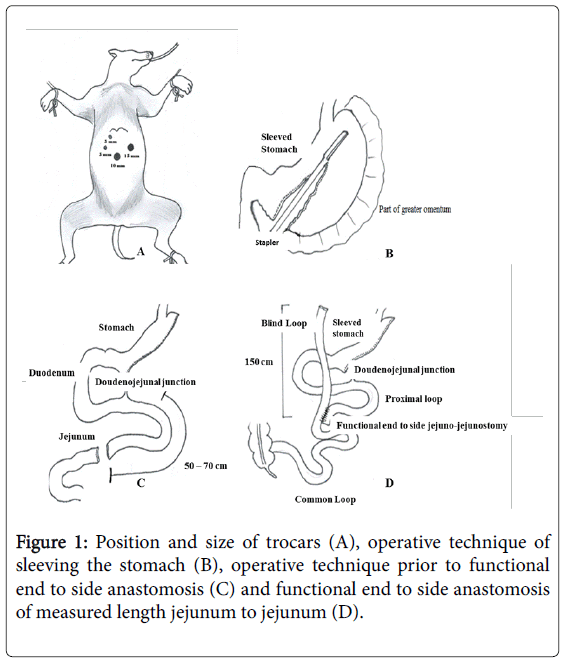
Gastric sleeve procedure: After sterile skin preparation with povidine iodine, a pneumoperitoneum with carbon dioxide was established. A 12 mm optical trocar was placed under direct vision approximately 15 cm below the xiphoid process and 3 cm to the left of midline. A 450 angled laparoscope was placed through the port into the peritoneal cavity and 12 mm port was placed in the left lateral flank, medial to the edge of the colon in a supine position and at the same level as per umbilical port. Next, a 5 mm trocar port was placed along the left subcostal margin between the xiphoid process and the left flank port. Another 12 mm port was placed in the right epigastric region and a fourth 12 mm port was placed in the mid-epigastric region caudal and medial to the previous port (Figure 1A).
The pylorus of the stomach was identified and the greater curve of the stomach elevated. A ligasure was used to enter the greater sac via division of the greater omentum. The greater curvature of the stomach was dissected free from the omentum and short gastric blood vessels using the ligasure. The dissection was started 5 cm from the pylorus to the Angle of His and a 10 mm caliber was then passed through the esophagus, stomach, and into the first portion of the duodenum. The caliber was aligned along the lesser curvature of the stomach and used as a template to perform the vertical sleeve gastrectomy beginning 2 cm proximal to the pylorus and extending to the Angle of His (Figure 1B).
An endoscopic linear cutting stapler was used to serially staple and transect the stomach just to the left and lateral to the caliber. The transacted stomach, which includes the greater curvature, was completely freed and removed from the peritoneum through the left flank port incision. The staple line along the remaining tabularized stomach was tested for any leak by injecting methylene blue through the nasogastric tube. The staple line was concurrently evaluated for bleeding intraperitonealy with the laparoscope. A 19 French Blake drain was carved in the upper left quadrant along the sleeve gastrectomy staple line. The fascia of the left flank port site was closed with an absorbable suture on a trans-abdominal suture passer to prevent bowel herniation but did not close the fascia defects at the remaining port sites (Figure 1C).
Sleeve bypass: The greater omentum was dissected centrally by using ligasure to release any possible pressure that may occur during anastomosis. The ligament of trietz was identified (doudenojejunal junction) and at 70 cm distal, folded the bowel to obtain proximal and distal segment by using the stapler. Approximately 150 cm from the distal end of the dissected bowel, we brought the proximal dissected bowel segment and anastomosed it to the marked area of the bowel by performing functional end to side anastomosis using the stapler (Figure 1D).
A portion of the stomach tissue and intestinal tissue were collected as the surgery was being performed and processed for histological, Immunocytochemical and biochemical analysis. The same process was reaped at 6 month period when canine were sacrificed.
Biochemical analyses
The stomach and intestinal tissue from each animal at the time of surgery and after 6-months post-operative were obtained and their weights were recorded. The tissues were homogenized in cold potassium phosphate buffer (0.05 M, pH 7.4). The homogenates were centrifuged at 5000 rpm for 10 min at 4°C. The resulting supernatant was used for determination of malondialdehyde (MDA), reduced glutathione (GSH), and nitric oxide (NO) levels, and catalase enzyme activity using commercial colorimetric assay kits.
Determination of MDA level: The levels of MDA in the stomach and intestinal homogenates were measured as suggested by the manufacturer, measuring absorbance at 534 nm (ZellBio GmbH, Germany).
Determination of GSH level: The stomach and intestinal GSH levels were measured using commercial colorimetric assay kit following manufacturer’s recommendations, measuring absorbance at 405 nm (ZellBio GmbH, Germany).
Determination of NO level: The stomach and intestinal NO levels were measured using commercial colorimetric assay kit, measuring absorbance at 540nm (abcam, USA).
Determination of catalase enzyme activity: The activity of catalase enzyme in the stomach and intestinal homogenates were measured using commercial colorimetric assay kit (Sigma-Aldrich, USA). The assay was performed according to manufacturer’s recommendations. One unit of catalase is defined as the amount of the enzyme required to decompose 1 μM of H2O2 per minute.
Histological and Immunocytochemical analysis
The stomach and intestinal tissues obtained from each animal were fixed in 10% formalin solution, dehydrated in ascending grades of ethanol and embedded in paraffin. Sections at 4 μm thickness were taken, stained with hematoxylin and eosin (H&E) and examined under light microscope. Four μm thick sections of the stomach and intestinal tissues were deparaffinised, rehydrated, and endogenous peroxidase activity was blocked with 3% H2O2 in methanol. Sections were pretreated in citrate buffer (pH 6.0) in a microwave. Sections were incubated with polyclonal antibodies specific for the dog targets.
The antibodies used were anti-nitric oxide synthase (iNOS), anticyclooxygenase- 2 (COX-2), anti-Fas ligand (FasL), and anti-caspase-3 antibodies ( Thermo Scientific, USA, dilution 1:1000), and anti-tumor necrosis factor-α (TNF-α) (US Biologicals, USA, dilution 1:1000). The sections were incubated with biotinylated goat anti-polyvalent, then with streptavidin peroxidase and finally with diaminobenzedine plus chromogen. Slides were counterstained with hematoxylin and visualized under light microscope and the extent of cell immunopositivity was assessed.
Statistical analysis
The results were analyzed by t-test using GraphPad InStat (version 3.1). P<0.05 was selected as the criterion for statistical significance.
Results
Surgery and weight loss
All surgical procedures were successfully performed with no deaths or complications. There was no post-surgical fluid collection, abscesses or adhesion to the surrounding organs. All peritoneal and blind loop cultures were negative. No post-operative leaks were detected. The single important observation of this study was that all the dogs had significant weight loss after surgery and this loss were maintained up to 6 months, till the dogs were sacrificed. The average weight loss was approximately 34% (Table 1).
| Pre-operative body weight | Post-operative body weight | Loss of Weight |
|---|---|---|
| 25.705 ± 1.24 kg | 16.6 ± 1.17 kg* | (-8.805 ± 0.97) kg, (34%) |
Table 1: Results of weight loss due to sleeve surgery, the loss in weight is considered significant (*P<0.05) pre-operative body weight compared to post-operative body weight (n=10).
Biochemical analyses (oxidative stress)
Malondialdehyde (MDA) has been widely used as a biomarker for lipid peroxidation. From our results, in the postoperative stomach tissue the level of MDA had gone up by 35%, a significant increase as compared to pre-operative control (124.01 ± 7.9 vs. 62.39 ± 3.8 nmol/ gm). However in the blind intestinal loop there was no such significant change (Table 2).
| MDA (nmol/g tissue) | |||
|---|---|---|---|
| Stomach | Blind loop Intestine | ||
| Pre-operative | Post-operative | Pre-operative | Post-operative |
| 62.39 ± 3.8 | 124.01 ± 7.9* | 87.55 ± 5.6 | 83.30 ± 8.3 |
| GSH (mmol/g tissue) | |||
| Stomach | Blind loop Intestine | ||
| Pre-operative | Post-operative | Pre-operative | Post-operative |
| 0.73 ± 0.07 | 0.79 ± 0.16 | 1.05 ± 0.11 | 0.82 ± 0.07* |
| NO (nmol/100 mg tissue) | |||
| Stomach | Blind loop Intestine | ||
| Pre-operative | Post-operative | Pre-operative | Post-operative |
| 149.96 ± 13.1 | 196.34 ± 15.1* | 56.91 ± 9.21 | 66.46 ± 7.41 |
| Catalase (U/g tissue) | |||
| Stomach | Blind loop Intestine | ||
| Pre-operative | Post-operative | Pre-operative | Post-operative |
| 1.97 ± 0.73 | 3.90 ± 0.24* | 0.67 ± 0.16 | 0.10 ± 0.04* |
Table 2: Results of pre-operative and post-operative Malondialdehyde (MDA), Glutathione (GSH), nitric oxide (NO) and Catalase levels in the stomach and intestine of the experimental dogs; *P<0.05 considered significant comparing pre and post-operative value (n=10).
Glutathione (GSH) levels were unchanged in the post-operative stomach but exhibited a significant decrease (1.05 ± 0.11 vs. 0.82 ± 0.07 nmol/gm) in the post-operative blind loop intestine (Table 2).
Nitrous Oxide (NO) levels in post-operative stomach are again significantly elevated (196.34 ± 15.1 vs. 149.96 ± 13.1 nmol/100 mg of tissue) while in blind loop intestines no significant change in the level of NO was observed (Table 2).
Catalase enzyme, an oxidative stress marker was significantly elevated in post-operative stomach tissue (3.90 ± 0.24 vs. 1.97 ± 0.73 U/gm. of tissue) However, in blind intestinal loop its activity (0.10 ± 0.04 vs. 0.67 ± 0.16 U/gm. of tissue) was significantly inhibited (Table 2).
Histology of stomach and blind intestinal loop
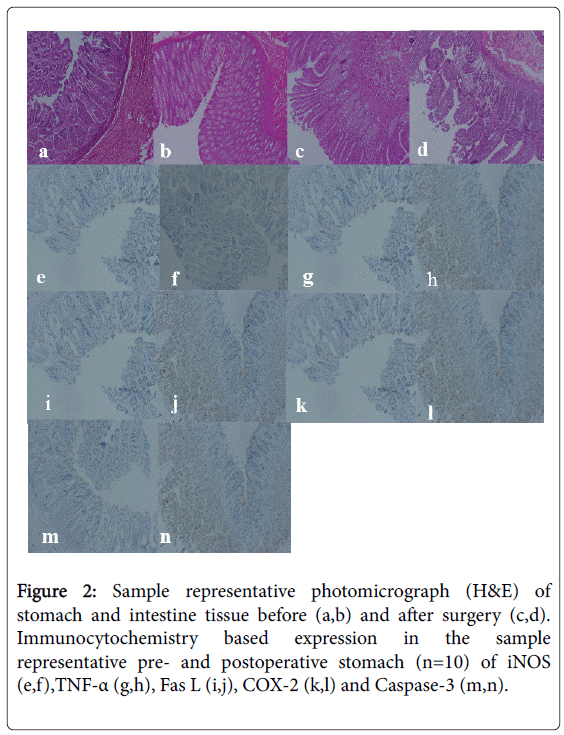
Histology of stomach and intestine tissues before (Figures 2a and 2b) and after surgical procedure for stomach and blind intestinal loop (Figures 2c and 2d) did not exhibit any significant changes, suggesting normal histology in pre-and post-operative tissues.
Figure 2: Sample representative photomicrograph (H&E) of stomach and intestine tissue before (a,b) and after surgery (c,d). Immunocytochemistry based expression in the sample representative pre- and postoperative stomach (n=10) of iNOS (e,f),TNF-α (g,h), Fas L (i,j), COX-2 (k,l) and Caspase-3 (m,n).
Immunocytochemistry studies
Immunocytochemical staining was performed on the tissues from stomach (pre and post-operative, Figures 2a-2d) for inducible nitric oxide synthase (iNOS, Figures 2e and 2f), Tumor necrosis factor-α (TNF-α Figures 2g and 2h), Fas ligand (Fas L, Figures 2i and 2j), cyclooxygenase-2 (COX-2, Figures 2k and 2l) and Caspase-3 (Figures 2m and 2n). All these ligands and enzymes exhibited increased expression in the postoperative stomach tissue as compared to preoperative stomach tissue. The increased expression can be visualized from the brown color of the postoperative stomach tissue not present in the preoperative stomach tissue. The expression of these ligands and enzymes were not quantitated. The presence of these above ligands and enzymes were also tested in blind intestinal loop postoperative tissue, however there was no difference between the pre and postoperative expression (data not shown).
Discussion
Improvement in biochemical profile for diabetes and other metabolic disorders is one of the main objectives of bariatric surgery. The metabolic effects, weight loss and improvement in quality of life have been reported following bariatric surgery [9-10]. Recent advances in bariatric surgery have made it safer with minimal perioperative mortality risk associated with laparoscopic gastric banding surgery [1]. Because the function of pylorus is maintained, there is less restriction on what patients can eat, compared to other gastric bypass procedures. Also it has been reported that patients experience less hunger after sleeve gastrectomy in comparison to gastric band or gastric bypass, may be due to removal of large part of stomach that secrets hormones [1,6]. In a recent report on long term follow-up outcome of laparoscopic sleeve gastrectomy and gastric bypass procedures, shows that bariatric surgery is more effective than medical treatment in weight reduction and diabetic control. However, survival benefits adverse outcome is not clear [27].
In our studies, we took out 70-80% of the stomach along the greater curvature. In the second step, approximately 150 cm of the proximal part of the small intestine was rendered unavailable to the absorption process. In our procedure, compared to other sleeve surgeries, we left behind part of small intestine with the purpose to minimize trauma of extensive surgery and future possible re-use of the part. Our results show that there is significant weight loss that was maintained up to six months post-operation. Bariatric surgery has been shown to produce much greater and sustained weight loss when compared to conventional methods. However, various markers of inflammation, CRP, TNF-α and IL-6 have been reported to be affected in bariatric surgery [15]. Though CRP is known to decrease with weight loss, the changes in the other markers are not clear. In our studies, we determined TNF-α, iNOS, FasL, COX-2 and Catalase expression by immunocytochemistry and found their expression increased in postoperative stomach tissue as compared to preoperative stomach. There was no change of these markers in the postoperative blind loop intestinal tissues, suggesting that blind loop did not undergo inflammatory process.
We evaluated the oxidative stress due to surgery. Oxidative stress markers such as MDA, one of the most frequently used indicator of cell membrane injury was significantly elevated in post-operative stomach but not in post-operative blind loop intestine. Surgical intervention per se elicits inflammatory response with cytokine activation and overproduction of oxidative stress markers. This observation would suggest that extensive stomach surgery resulted in over production of oxidative stress as compared to lesser stress in the blind loop intestine due to minimal surgery. Low glutathione (GSH) have been implicated with decreased cellular antioxidant capacity, in the pre- and postoperative stomach tissues there is no significant change in its levels. However, in the postoperative blind intestinal loop there is significant decrease in GSH levels and thus may suggest that thiolredox status is altered. Our results further suggest that there is an increase in the expression of anti-oxidant enzymes such as Nitric oxide synthase measured as nitric oxide and significant increase in MDA, NO and catalase levels in post-operative stomach even after six months, suggesting that antioxidant state that existed pre-operative stage is not restored at six months of post-surgery. Most of the reports on the elevated oxidative stress and inflammatory response in obese subjects subjected to RYGB has been determined in the circulating plasma and other biological fluids and found to improve significantly after surgery. However, we have not determined these markers in the plasma, instead we were interested to determine if there is any improvement of these markers at the source in the stomach and blind loop intestinal tissue after 6 months surgery. We did observe elevated levels of oxidative stress and inflammatory markers at six months in these tissues, suggestive that in the local tissue it may take longer than six months for these markers to return to normal healthy levels.
In conclusion, as a result of our surgical procedure, there is a significant weight loss at six months postoperative and most of the oxidative markers are still elevated at this time point. This is also true with inflammatory markers. Our investigation is one time point study of these markers and long term implication is not known. However, in recent study on pro-inflammatory and oxidative stress markers in human subjects undergone Roux-en-Y gastric bypass, suggests that it takes more than one year postoperatively for inflammatory and antioxidant markers to return to preoperative status [28].
Disclaimer
The authors declare no financial or commercial conflict of interest related to this publication.
Acknowledgements
We are thankful to Mr. Abdul Razak for technical assistance. This study was supported by the research grant from the Deanship of Scientific Research at King Faisal University, Al-Hasa, Kingdom of Saudi Arabia.
References
- Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK (2008) Weight loss, appetite suppression and changes in fasting and postprandial ghrelin and peptide-Y levels after Roux-en-Y gastric bypass and sleeve gastrectomy: A prospective and double blind study. Ann Surg 247: 401-407.
- DeMaria EJ, Pate V, Warthen M, Winegar DA (2010) Baseline data from american society for metabolic and bariatric surgery-designated bariatric surgery centers of excellence using the bariatric outcomes longitudinal database. Surg Obes Related Dis 6: 347-355.
- Gagner M, Matteotti R (2005) Laparoscopic biliopancreatic diversion with duodenal switch. Surg Clin North Am 85: 141-149.
- Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, et al. (2013) The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 257: 791-797.
- O’Brien PE (2015) Controversies in bariatric surgery. Br J Surg 102: 611-618.
- Himpens J, Dapri G, Cadiere GB (2006) A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: Results after 1 and 3 years. Obes Surg 16: 1450-1456.
- Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, et al. (2009) Remission of type diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis 5: 305-309.
- Dixon JB, le Roux CW, Rubino F, Zimmet P (2012) Bariatric surgery for type 2 diabetes. Lancet 379: 2300-2311.
- Kiong KL, Ganesh R, Cheng AKS, Lekshiminarayanan R, Lim SC (2010) Early improvement in type 2 diabetes mellitus post Roux-en-Y gastric bypass in Asian patients. Singapore Med J 51: 937-943.
- Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, et al. (2009) Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 33: 786-795.
- Thaler JP, Cummings DE (2009) Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150: 2518-2525.
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, et al. (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623-1630.
- Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, et al. (2011) Early Improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg 21: 896-901.
- Laferrère B, Heshka BS, Wang K, Khan Y, McGinty J, et al. (2007) Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30: 1709-1716.
- Bose MM, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B (2009) Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg 19: 217-229.
- Basso N, Capoccia D, Rizzello M, Abbatini F, Mariani P, et al. (2011) First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: The gastric hypothesis. Surg Endosc 25: 3540-3550.
- Korner J, Bessler JM, Cirilo LJ, Conwell IM, Daud A, et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90: 359-365.
- Li JV, Ashrafian H, Bueter HM, Kinross J, Sands C, et al. (2011) Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 60: 1214-1223.
- Faintuch JJ, Ishida RK, Jacab M, Ribeiro AS, Kuga R, et al. (2007) Increased gastric cytokine production after Roux-en-Y gastric bypass for morbid obesity. Arch Surg 142: 962-968.
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, et al. (2006) Interleukin-23 drives innate and T cell– mediated Intestinal inflammation. J Exp Med 203: 2473-2483.
- Paajanen L, Kokkonen J, Karttunen TJ, Tuure T, Korpela R, et al. (2006) Intestinal Cytokine mRNA expression in delayed-type cow’s milk allergy. J Pediatr Gastroenterol Nutr 43: 470-476.
- Umehara Y, Kudo M, Nakaoka R, Kawasaki T, Shiomi M (2006) Serum proinflammatory cytokines and adhesion molecules in ulcerative colitis. Hepatogastroenterology 53: 879-882.
- Miller GD, Nicklas BJ, Fernandez A (2011) Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 7: 618-624.
- Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, et al. (2016) Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes (Lond) 40: 275-280.
- João CE, Valezi AC, Delfino VDA, Lavado EL, Barbosa DS (2010) Reduction in plasma levels of inflammatory and oxidative stress indicators after Roux-En-Y gastric bypass. Obes Surg 20: 42-49.
- Monte SV, Caruana JA, Ghanim H, Sia CL, Korzeniewski K, et al. (2012) Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery 151: 587-593.
- Hsu CC, Almulaifi A, Chen JC, Ser KH, Chen SC, et al. (2015) Effect of bariatric surgery vs. medical treatment on type 2 diabetes in patients with body mass index lower than 35: Five-year outcomes. JAMA Surg 150: 1117-1124.
- da Silva VR, Moreira EA, Wilhelm-Filho D, de Miranda JX, Benincá J, et al. (2012) Proinflammatory and oxidative stress markers in patients submitted to Roux-en-Y gastric bypass after 1 year of follow-up. Eur J Clin Nutr 66: 891-899.
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 6420
- [From(publication date):
June-2017 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 5465
- PDF downloads : 955