Research Article Open Access
Solution and In silico Ligand Binding Studies of Cicer arietinum Lectin
| Madhurima S. Wakankar1, Krunal A. Patel2, Musti V. Krishnasastry3 and Sushama M. Gaikwad1* | |
| 1Division of Biochemical Sciences, National Chemical Laboratory, Pune, Maharashtra, 411008, India | |
| 2Division of Plant Tissue Culture, National Chemical Laboratory, Pune, Maharashtra, 411008, India | |
| 3National Centre for Cell Science, Pune, Maharashtra, 411007, India | |
| *Corresponding Author : | Dr. Sushama M. Gaikwad Division of Biochemical Sciences National Chemical Laboratory Pune-411008, Maharashtra, India Tel: +912025902241 Fax: +912025902648 Email: sm.gaikwad@ncl.res.in |
| Received December 28, 2012; Accepted January 07, 2013; Published January 10, 2013 | |
| Citation: Wakankar MS, Patel KA, Krishnasastry MV, Gaikwad SM (2013) Solution and In silico Ligand Binding Studies of Cicer arietinum Lectin. Biochem Physiol S2:002. doi:10.4172/2168-9652.S2-002 | |
| Copyright: © 2013 Wakankar MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The recombinant lectin from Cicer arietinum (rCAL) showed complex sugar specificity and could bind only the asialo triantennary glycan from Fetuin. The thermodynamic study of binding to this glycan indicated the process to be spontaneous and exothermic. The values obtained were, ΔG as -28.56 kJ mol-1; ΔH as -43.65 kJ mol-1 and ΔS as -50.65 Jmol-1K-1 at 25°C. The presence of four hemopexin-binding domains in the gene sequence indicated possible binding to hemin. Binding of hemin as studied by fluorescence spectroscopy, yielded an association constant of 3.55 x 107 M-1. The lectin also bound spermine and thiamine with association constants of 1.55 x 104 M-1 and 5.37 x 103 M-1, respectively. In silico investigation was carried out by protein-ligand docking using AutoDock Vina software. Binding energies were calculated for each ligand and the amino acids involved in the interaction of these ligands with the rCAL homology model were identified. ASN-8 residue was found to be important in binding of hemin and spermine to rCAL.
| Keywords |
| Chick pea lectin; Asialo triantennary fetuin; Hemin; Spermine; Thiamine; Docking |
| Introduction |
| The term “lectins” identifies proteins of non-immune origin which reversibly bind carbohydrates of glycoproteins, glycolipids, or polysaccharides with high affinity [1]. This high affinity and specificity for glycoconjugates has found many applications in biological and biomedical research [2]. Moreover, they share common structural characteristics of their binding sites and the mechanism of their ligand recognition, despite the broad variations in their specificities [3]. Consequently, they have been extensively studied as paradigms of protein-carbohydrate interactions. |
| In recent years, the drug discovery process has benefited significantly from computational studies on protein-carbohydrate interactions. Lectins have been successfully used in pharmaceutical applications as a system for drug targeting [4] and drug delivery [5]. For example, the Bacillus anthracis tetrasaccharide has been promoted as a promising lead for an anthrax vaccine [6]. |
| A lectin showing complex sugar specificity was purified in our laboratory from the seeds of Cicer arietinum according to the method of Katre et al. [7]. The N-terminal amino acid sequence of this lectin showed maximum identity (90%) with the first 25 amino acids of the major seed albumin (PA2) from Pisum sativum. The lectin gene was cloned using primers designed from the PA2 albumin, and the recombinant Cicer arietinum lectin (rCAL) was purified (methodology - communicated elsewhere). The homology with the PA2 albumin led us to investigate other albumin-like properties of this lectin. rCAL was found to contain four conserved hemopexin-like repeats similar to PA2 [8]. Proteins containing the hemopexin domain bind heme with great affinity. The functional presence of hemopexin-binding domains can be confirmed by the actual binding of hemin to the protein. Homologous albumins from plant seeds have been shown to bind hemin and spermine [9,10], as well as thiamine [11]. |
| The present paper describes the functional characterization of recombinant Cicer arietinum lectin with respect to its carbohydrate and ligand binding properties. The binding studies of rCAL with hemin, spermine and thiamine were carried out using fluorescence spectroscopy as well as protein-ligand docking. Residues of the protein involved in the interaction with these ligands were identified from the docking simulations. Binding energies for these interactions were obtained from both the techniques. |
| Materials and Methods |
| Materials |
| Cultivar BDN 9-3 of chick pea seeds was obtained from Badnapur Agricultural University, Jalna, India. Pronase-E, carboxypeptidase, aminopeptidase and neuraminidase enzymes and Fetuin, hemin, spermine and thiamine were obtained from Sigma-Aldrich, India. All the simple and complex sugars as well as general chemicals and buffers were procured from Sigma-Aldrich, India. Rabbit blood for the hemagglutination assay was obtained from the animal house of the National Institute of Virology, Pune, India. |
| Protein Preparation |
| The lectin gene from Cicer arietinum seeds was cloned in pET 28a+ vector and expressed in the BL21 CodonPlus (DE3)-RIL cells of E. coli. The recombinant protein was purified from the induced cell lysates by ion-exchange (unadsorbed on DEAE-cellulose) and gel filtration chromatography (Superose 12 prep grade, FPLC) [cloning and purification methodology – unpublished data, communicated elsewhere]. This purified lectin, rCAL, was the subject of the present study. |
| Preparation of asialo triantennary glycan |
| Triantennary-N-glycan was prepared from Fetuin. One gram of the glycoprotein was dissolved in 100 ml of 20 mM Tris-HCl (containing 150 mM NaCl, 0.5 % w/v sodium azide), pH 7.2 and digested by 50 mg of pronase-E at 37°C for 72 h; 20 mg of pronase-E was added after every 24 h. The digest was lyophilized, dissolved in 5 ml of 100 mM acetic acid, centrifuged (10,000 g, 20 min), supernatant was collected. The pellet was re-extracted five times in 1 ml of 100 mM acetic acid. Two ml of clear supernatant was chromatographed on Sephadex G-25 column (1.5×100 cm) pre-equilibrated with 20 mM acetic acid, and eluted with the same buffer at a flow rate of 20 ml/hour. The fractions (2 ml) were collected and those showing the presence of sugar were pooled and further digested by carboxypeptidase (10 U at pH 7.0 and 25°C for 24 h) and aminopeptidase (10 U at pH 8.5 and 25°C for 24 h). The residual peptides were removed by chromatography on a Dowex-50 column (1.5×4 cm) in 20 mM acetic acid [12,13]. The glycopeptides were desialated by incubating with 5 U of neuraminidase in 20 mM Tris-HCl buffer (pH 7.2) at 37°C for 4 h; the enzyme and sialic acid were removed by successive chromatography on Sephadex G-25 (1.5×10 cm) and Dowex-50 as described above. |
| Protein concentrations were determined according to the method of Lowry et al. [14] using bovine serum albumin (BSA) as standard. The total neutral sugar content was determined by phenol-sulphuric acid method of Dubois et al. [15] using mannose as the standard. |
| Hemagglutination assay |
| Rabbit erythrocytes were washed 5 times with PBS (phosphate-buffered saline, 20 mM potassium phosphate buffer, pH 7.2, containing 150 mM NaCl). A 3% (v/v) suspension of the erythrocytes was prepared in the same buffer. Hemagglutination assays were performed according to Gurjar et al. [16]. |
| Hemagglutination inhibition assays |
| Hemagglutination inhibition assays were performed similarly, except that serially diluted sugar solutions (25 μl from 0.5 M stock) were pre-incubated for 15 min at 27°C with 25 μl (600 ng) of the lectin. Erythrocyte suspension (50 μl) was then added, mixed and the plates read after one hour. |
| Fluorescence measurements and ligand-binding data analysis |
| Fluorescence measurements were carried out on a Perkin Elmer LS-50B spectrofluorimeter, with slit widths of 7 nm for both the monochromators and scan speed of 100 nm/min. The baseline was corrected by subtracting the contribution of the buffer. Chick pea lectin samples (150 μg/ml) in 20 mM phosphate buffer, pH 7.2 were placed in a quartz cuvette maintained at desired temperature (± 0.1°C) by means of a Julabo circulating water bath. The sugar/ligand solution was added in 10-12 aliquots (5 to 10 μl each). A 1.8 mM stock concentration of the asialo triantennary glycan from fetuin was used. The ligands were used in the concentration of 10 μM (for Hemin) and 5 mM (for Spermine and Thiamine). Samples were excited at 295 nm and the emission spectra were recorded in the range of 310 nm to 400 nm. Each spectrum was an average of 5 accumulations. The fluorescence intensity at 343 nm (λmax of the lectin) was considered for further analysis. Corrections were also made to compensate the dilution effect upon addition of sugar/ligand to the lectin. At the highest concentration of the sugar/ligand to lectin, volume change was less than 5% of the solution in the cuvette. Each data point was an average of three independent sets of experiments with SD less than 5%. |
| The association constants were calculated according to the method described by Chipman et al. [17]. The abscissa intercept of the plot of log [C]f against log {(ΔF)/(Fc– F∞)}, where [C]f is the free ligand concentration, yielded pKa value for lectin-ligand interaction according to the relationship: |
|
log [Fo−Fc/ Fc − F∞] = log Ka + log {[C]t – [P]t (ΓΆΒ?Β?F/ΓΆΒ?Β?F∞)} (1) |
| where Fc is the fluorescence intensity of the lectin at any point during the titration, [P]t is the total protein concentration, ΔF∞ is the change in fluorescence intensity at saturation binding, [C]t is the total ligand concentration, and [C]f is the free ligand concentration, given by, |
| [C]f = {[C]t − [P]t (ΔF/ΔF∞)} (2) |
| Free energy changes of association (ΔG) were determined by the equation, |
| ΔG = -RT lnKa (3) |
| Temperature dependence of the association constants was used to determine the thermodynamic parameters. Changes in enthalpy (ΔH) were determined from the Van’t Hoff plots by using the equation, |
| lnKa = (-ΔH/RT) + ΔS/R (4) |
| where ΔH is enthalpy change, R is gas constant, ΔS is entropy change and T is the absolute temperature. The entropy change was obtained from the equation, |
| ΔG = ΔH - TΔS (5) |
| Comparative modeling of rCAL |
| To build the three-dimensional model of rCAL, protein sequences of the closest homologues with known crystal structures were selected from the PDB. The 3D models of rCAL were built with the MODELLER 9v10 program [model construction and validation – unpublished data, communicated elsewhere]. |
| Docking studies of rCAL model with ligands |
| The AutoDock Vina 1.1.2 [18] docking pacKage was used for ligand flexible docking simulations using the default settings. The structures of all the ligands were obtained from PubChem (http://pubchem.ncbi.nlm.nih.gov/) database. The pdbqt files of ligands and receptor were generated using the MGLTools [19]. The pdbqt file is a modified protein data bank file format containing the information of atomic charges, atom type definitions and, for ligands, topological information. The grid box was defined from the binding sequences reported for hemin and spermine in the crystal structure of LS-24 (PDB ID: 3LP9). The size of the docking grid was kept as 32 Å×32 Å × 32 Å and the grid spacing set at 1 Å. The interactions of the ligands with protein were visualized in PyMOL 1.3 (www.pymol.org) and LIGPLOT v 4.5.3 [20]. The binding energy was obtained for each ligand. The lowest binding energy conformation was considered as the most favourable docking pose. |
| Results |
| The specificity of the purified recombinant Cicer arietinum lectin for simple sugars and complex carbohydrates was checked by hemagglutination inhibition assay. As a first step towards investigating its biological role, binding of rCAL with hemin, spermine and thiamine was examined using fluorescence spectroscopy. |
| Hemagglutination and hemagglutination inhibition assay |
| The lectin showed hemagglutination activity only against pronase-treated rabbit erythrocytes. The hemagglutination titre was obtained to be 5 × 103 hemagglutination units per mg of protein. Simple sugars like glucose, mannose, galactose, rhamnose, xylose, fucose, raffinose, trehalose, lactose, glucosamine, mannosamine, galactosamine, methyl-α-glucose, methyl-β-mannose, methyl-α-galactose, N-acetyl-mannosamine, N-acetyl-galactosamine, N-acetyl-D-glucosamine, D-mannitol, D-glucuronic acid, D-polygalacturonic acid and D-glucose pentaacetate failed to inhibit the hemagglutinating activity of the lectin. Hemagglutination by rCAL was inhibited by glycoproteins viz. fetuin, asialofetuin, and its asialotriantennary glycans. The minimum inhibitory concentration for fetuin and its asialo glycan was found to be 32 μg. Lectins recognizing only complex glycans have been reported earlier. For example, the lectin from wild cobra lily, Arisaema flavum [21] shows inhibition only for asialo fetuin with an inhibitory concentration of 250 μg/ml. |
| Ligand binding with Asialo triantennary glycan from Fetuin |
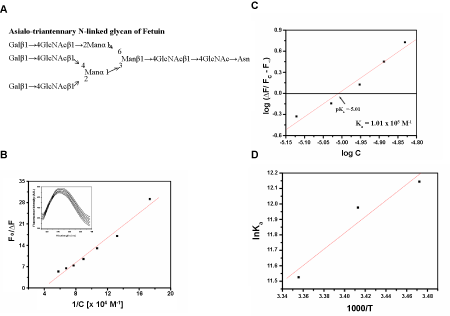
| Fluorescence titration of the rCAL with N-linked asialo triantennary glycan prepared from fetuin structure shown (Figure 1A) resulted in quenching of the lectin fluorescence (Figure 1B). The value of F∞ was derived from the plot shown in figure 1B. The binding constant Ka of 1.01 × 105 M-1 at 25°C was determined from the plot of log [ΔF ⁄ (Fc - F∞], versus log [C]f (Figure 1C). Binding was also checked at 15°C (Ka, 1.88×105 M-1) and 20°C (Ka, 1.59×105 M-1). A decrease in the association constant (Ka) was observed with increase in temperature. Thermodynamic parameters were calculated for the glycan binding. Van’t Hoff plots were linear (r > 0.9) in the range of temperatures studied (Figure 1D). The change in Gibb’s free energy (ΔG) was -28.56 kJmol-1 (±0.87), indicating binding to be spontaneous; the negative value of change in enthalpy (ΔH) of -43.65 kJmol-1 (±1.28) demonstrated the exothermic nature of the reaction. A negative change in entropy (ΔS) (-50.65 Jmol-1K-1). |
| (±1.48) may be due to the formation of more H-bonds between the glycan and the lectin. Few reports are available on lectins showing inhibition against complex glycans, like the lectin IV from Griffonia simplicifolia and PHA-L from P. vulgaris [22]. These belong to the group termed “complex” with specificity towards N-glycans. A stronger inhibition of rCAL activity by asialo fetuin than that with fetuin was observed. The interference due to sialic acid may be abolished by the action of neuraminidase, resulting in better interaction with rCAL (due to the exposure of the Galβ1→4GlcNAc residue). A similar observation has been made for the lectin from Fusarium solani, by Khan et al. [23], which interacts better with the asialo glycans of fetuin and fibrinogen than with simple galactose residue or its derivatives. The binding in this case is also enthalpically driven and an exothermic reaction. |
| Ligand binding |
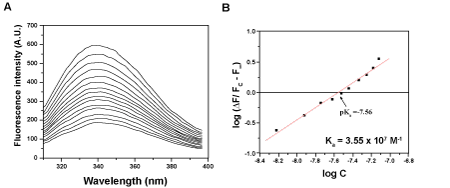
| Titration of rCAL with hemin at 25°C resulted in quenching of the fluorescence (Figure 2A). The slope of the plot of log [ΔF/Fc− F∞], versus log [C]f was near unity, hence one binding site per monomer of rCAL was predicted. The lectin bound hemin with an association constant, Ka=3.55 x 107 M-1 (Figure 2B). A similar quenching profile was observed when rCAL was titrated with 5 mM each of Spermine and Thiamine at 25°C. The association constants Ka obtained for spermine and thiamine binding were 1.55 × 104 M-1 and 5.37 × 103 M-1, respectively (Table 1). |
| Docking with ligands |
| MODELER 9v10 was used to construct a homology model for rCAL using the crystal structure of LS-24 albumin from Lathyrus sativus (PDB ID: 3LP9) as the template. |
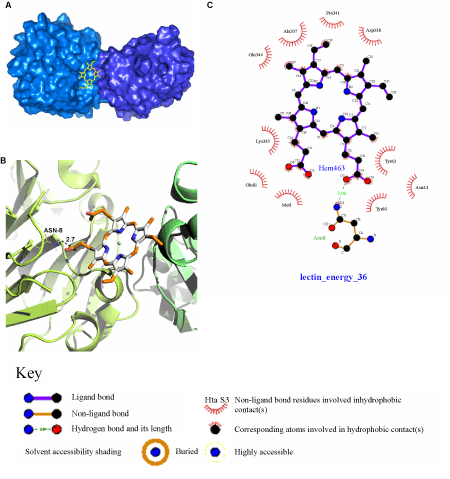
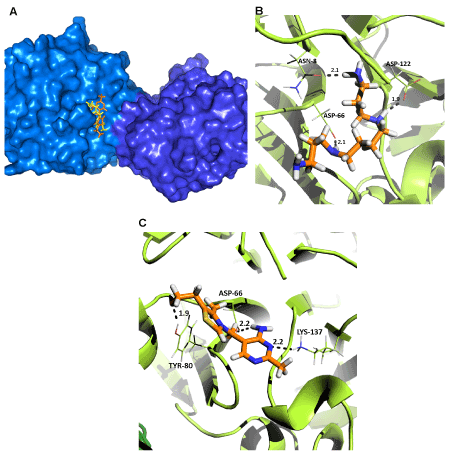
| The probable binding site for hemin was deduced in between the two monomers in the rCAL dimer model (Figure 3A), since it interacted with residues from both the monomers. A single amino acid (ASN-8) could be identified for the polar interaction between rCAL and hemin (Figure 3B). Hydrophobic interactions occurring between hemin and rCAL were deduced from the LIGPLOT. Residues involved were MET-1, ASN-24, TYR-63, TYR-80, GLU-81 (all from the A chain (231 residues per chain)), LYS-335 (B chain - 104), ALA-337 (B chain - 106), ASP-338 (B chain - 107), PRO-341 (B chain - 110) and GLU-344 (B chain - 113) (Figure 3C). The most favourable docked pose of hemin had a binding energy of -31.35 kJmol-1. For spermine and thiamine also, the binding sites on the model were present only on one of the two monomers and located close to each other (Figure 4A). Spermine interacted with amino acids ASN-8 (2.1 Å), ASP-66 (2.1 Å) and ASP-122 (1.9 Å) of rCAL by formation of hydrogen bonds (bond lengths given in brackets) (Figure 4B). Thiamine formed hydrogen bonds with ASP-66 (2.2 Å), TYR-80 (1.9 Å) and LYS-137 (2.2 Å) of rCAL (Figure 4C). Binding energies of -15.88 kJmol-1 and -22.15 kJmol-1 were obtained for spermine and thiamine, respectively. |
| Interestingly, both hemin and spermine were found to interact with ASN-8 of the protein. Gaur et al. [9] have shown that hemin and spermine bind to the LS-24 albumin in a mutually exclusive manner. Spermine is bound to the dimeric LS-24 albumin, but hemin binding results in dissociation of the subunits accompanied by loss in the ability to bind spermine. This binding property might be true for rCAL also, wherein a common residue is involved in the binding of both hemin and spermine. |
| In the crystal structure of LS-24, binding of spermine involves the interaction of GLU-80, ASN-81, and SER-118 [9]. In the rCAL model, spermine binding involves ASN and two ASP residues. As can be seen from figure 4B, the oxygen atoms from the side chains of ASN-8, ASP-66, and ASP-122 are involved in hydrogen bonding with the hydrogen atoms of the spermine ligand. Hence, the replacement of Serine in the template to Aspartate does not affect the binding of spermine in the model. The binding pocket formed for spermine in the model of rCAL is different from that formed in the crystal structure of LS-24. The rCAL sequence has LYS residue in the 82nd position. In the model, the side chains of this LYS-82 residue from both monomers protrude in such a way that they occupy a position in the intersection (in between) of the two monomers (this is the site where spermine is shown to bind in the crystal structure of LS-24). Hence, this site is unavailable for the binding of spermine in the model. |
| Discussion |
| The recombinant chick pea lectin (rCAL) was found to bind the asialo triantennary fetuin glycan and the reaction was spontaneous and exothermic. Since rCAL exhibited complex sugar specificity, investigation of the lectin for possible occurrence of binding sites for other bio molecules was explored. The nucleotide sequence of rCAL revealed the presence of the hemopexin fold. The hemopexin fold has already been reported from seed albumins of Pisum sativum [8], Lathyrus sativus [9], Lens culinaris [10] and Vigna unguiculata [24]. Hemin could strongly bind rCAL as seen from fluorescence spectroscopy, confirming the functional presence of the hemopexin domains. Not all proteins containing the hemopexin fold bind hemin, as is the case with the CP4 protein from Vigna unguiculata [24]. rCAL also showed binding to spermine and thiamine. Plant hemopexins have been shown to be involved in spermine biosynthesis [25]. Albumins from pea and chick pea bind thiamine, hence they have been thought to be involved in storage of the vitamin during germination [11]. Both spermine and thiamine have been shown to play important roles in reducing oxidative stress during different abiotic stress conditions [26,27]. Taken together, these studies lead to the speculation of a possible role of rCAL in plant defence against various stress conditions. Docking simulations could help in the identification of the residues involved in the interaction of these ligands with the rCAL homology model. |
| Acknowledgements |
| This paper is dedicated to the memory of our dear colleague Late Dr. M. Islam Khan who initiated this work. The authors are thankful to Late Mr. Rohtas Rangera for his help. MSW and KAP are supported by Senior Research Fellowships from the CSIR, India. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14094
- [From(publication date):
specialissue-2013 - Aug 15, 2025] - Breakdown by view type
- HTML page views : 9487
- PDF downloads : 4607
