Review Article Open Access
Spanish Ancient Wheats: A Genetic Resource for Wheat Quality Breeding
| Juan B. Alvarez* and Carlos Guzmán | ||
| Department of Genetics, School of Agricultural and Forestry Engineering, Building Gregor Mendel, Rabanales Campus, University of Córdoba, ceiA3, ES-14071, Cordoba, Spain | ||
| Corresponding Author : | Juan B. Alvarez Department of Genetics, School of Agricultural and Forestry Engineering Building Gregor Mendel, Rabanales Campus University of Córdoba, ceiA3, ES-14071 Córdoba, Spain E-mail: jb.alvarez@uco.es |
|
| Received January 23, 2013; Accepted February 13, 2013; Published February 17, 2013 | ||
| Citation: Alvarez JB, Guzmán C (2013) Spanish Ancient Wheat: A Genetic Resource for Wheat Quality Breeding. Adv Crop Sci Tech 1:e101. doi: 10.4172/2329-8863.1000101 | ||
| Copyright: © 2013 Alvarez JB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Wheat (Triticum sp.) is one of the most important food crops worldwide. Di-, tetra- and hexaploid species are available, which along with the different subspecies of this genus have been used for food and feed. However, the genetic diversity of this crop has been seriously compromised, and only two main species; the durum and bread wheat, currently exist. The germplasm materials that were used in the past in Spain, have been regarded as ancient wheats, thus forming a group of neglected or underutilized crops that could be an interesting reservoir of variation for modern wheat breeding. In the last decade, our group has been evaluating and characterizing for the morphological and quality traits some of these Spanish ancient wheats. In these materials, using the SDS-PAGE technique and DNA sequencing, we detected an important variability for seed storage proteins, starch synthases and puroindolines, which are related with three important quality characteristics of wheat: gluten levels, starch levels and hardness, respectively. These novel variants could be used with the dual goal of genetic improvement and enriching the gene pool of these components in modern wheat.
| Keywords | |
| Diversity; Genetic resources; Morphological traits; Puroindolines; Seed storage proteins; Starch; Wheat | |
| Wheat: Origin and Use | |
| Among the first plants that were cultivated and domesticated, the characteristics associated with ease of transportation and storage was highly desired. The Gramineae species, and most importantly, their domestic variants, such as the cereals, (included in this type of plants) represent the staple food in different parts of the world. Their origin and domestication appear connected to the origin of the main civilizations. So, each one of the three main crops (rice, maize and wheat) have been associated with one important civilization: wheat with Sumer or Egypt in the Mediterranean region; rice with Chinese civilization in Asia; and maize with the Olmec and Maya civilizations in America [1]. | |
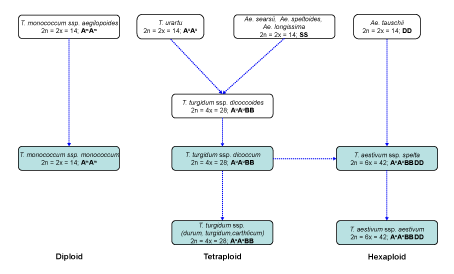
| The wheat origin and domestication started in the Near East, in the well-known zone regarded as the Fertile Crescent [2]. The wheat is a complex polyploid formed by multiple species of different ploidy levels, resulting from a combination of genomes from different species of the Triticeae tribe in the Poaceae family (Figure 1). Thus, diploid (2n=2×=14, AA), tetraploid (2n=4×=28, AABB), and hexaploid (2n=6×=42, AABBDD) species can be found. | |
| The origin of the A and D genomes is well known. T. urartu Thum ex. Gandil (2n=2×=14, AuAu), a wild diploid species, has been proposed as the donor of the A genome in the polyploid species of wheat [3]. With respect to the D genome, several studies suggest that the donor is Aegilops tauschii Coss. (2n=2×=14, DD) [4,5], crossed with cultivated emmer (Triticum turgidum spp. dicoccum Schrank em. Thell. 2n=4×=28, AuAuBB), followed by chromosome doubling, resulting in spelt (T. aestivum ssp. spelta L. em. Thell. 2n=6×=42, AuAuBBDD), the putative ancestor of bread wheat (T. aestivum ssp. aestivum L. em. Thell.), the species of the Triticum genus that is more important today. However, the origin of the B genome presents certain controversy. The currently accepted hypothesis suggests that T. urartu could have given rise to the wild tetraploid wheats, mainly in two different events. On one hand, a cross with an Aegilops species (section Sitopsis), probably Ae. speltoides Tausch. (2n=2×=14, SS), generated a wild emmer (T. turgidum ssp. dicoccoides Korn. ex Asch. & Graebner em. Thell., 2n=4×=28, AuAuBB), from which the cultivated emmer was derived through domestication. The rest of the tetraploid wheat, including durum wheat (T. turgidum ssp. durum Desf. em. Husn.), was derived from this species as well as the hexaploid wheat [6,7]. Similarly, the crossing of some other species of the section Sitopsis with T. urartu resulted in T. timophevii ssp. armeniacum Jakubz. em. Slageren (2n=4×=28, AuAuGG), whose domesticated form (T. timophevii ssp. timophevii) is restricted to western Georgia. | |
| Wheat Food Quality | |
| Wheat quality can be defined as the ability of a variety to produce flour suitable for a specific product. Consequently, this parameter is a variable that depends on consumer preferences, the product to be developed, and the process to be used in the preparation of the product. This trait is strongly associated with the grain components and their physico-chemical properties. | |
| Grain wheat is mainly composed of proteins (7-18%), lipids (1.5-2%) and carbohydrates (60-75%), together with other minor components such as certain vitamins and minerals [8]. Proteins and carbohydrates, especially starches, have considerable influence on three grain characteristics, closely linked to the technical wheat qualities required for baking or pasta manufacture. These are gluten strength, starch properties and grain hardness or texture, which are associated with the endosperm storage proteins, starch synthesis enzymes and puroindolines, respectively. | |
| Endosperm Storage Proteins | |
| Two main groups (gliadins and glutenins) have been identified among these proteins, according to their molecular characteristics [9]. Glutenins are also divided into high-molecular-weight (HMWGs) and low-molecular-weight (LMWGs) subunits [10,11]. The HMWGs are coded at the Glu-1 loci located on the long arm of group-1 homoeologous chromosomes, whereas on the short arm are located the Glu-3 loci that code for the LMWGs, and the Gli-1 loci that control the synthesis of ω-, γ- and some β-gliadins. On the short arm of group-6, homoeologous chromosomes are located the Gli-2 loci that code mainly for components present in the α region and some β-gliadins [12]. | |
| Different studies have indicated that the HMWGs are the major group of gluten proteins that determine the bread-making characteristics of dough, whereas the variation in LMWGs would be more important in pasta production [13]. With respect to the latter, most qualitative evaluations of LMWGs have been performed on B-type subunits, as they are the most abundant and easiest to detect, while very little is known about the role of C- and D-type subunits [14]. | |
| Starch Synthesis Enzymes | |
| The synthesis of starch in the endosperm occurs in the amyloplast, a plastid non- photosynthetic apparatus dedicated to this function. In this process, several enzymes act sequentially using ADP-glucose as a substrate by two different pathways, to synthesize the starch components (amylose and amylopectin). These enzymes are ADPglucose pyrophosphorylase, starch synthases (SS) and branching enzymes (BE) [15]. | |
| The most studied of the enzymes involved in starch synthesis are (GBSSI) Granule Bound Starch Synthase I or waxy protein, which is the key enzyme in amylose synthesis. Depending on the ploidy level of each species of wheat, one, two or three waxy proteins are present. The latter is the case for common wheat, where three waxy proteins are found encoded by the Wx-A1 gene (chromosome 7AS), Wx-B1 (chromosome 4 AL by translocation of a segment of chromosome 7BS), and Wx-D1 (7DS chromosome) [16]. The other enzyme that has been studied is the SGP-1 (SGP-A1, SGP-B1, and SGP-D1), which is coded by the SSIIa-A, SSIIa-B and SSIIa-D genes located on chromosome arms 7AS, 7BS and 7DS, respectively [17-19]. | |
| Puroindolines | |
| The molecular-genetic basis of wheat grain hardness is now well established, being related to “friabilin”, a Mr 15-kDa protein associated with the starch membrane, which is abundant in soft wheat starch, scarce in hard wheat starch, and absent in durum wheat [20]. The main components of “friabilin” are puroindolines a and b (Pina and Pinb); and a third minor protein called the grain softness protein (GSP). These proteins are encoded by the Pina-D1, Pinb-D1 and Gsp genes located in the locus Ha (Hardness), which is present in the short arm of the chromosome 5D of common wheat [21]. It is a complex locus formed by 10 closely linked genes [22], although only two loci (Pina-D1 and Pinb-D1) have so far been associated with grain hardness [23-26]. | |
| When both puroindolines are present in its functional form, grain texture is soft; while the alteration of any of them, either by gene deletion or inactivation, causes the grain texture to become harder [21,23,26-28]. At the extreme, grain texture is very hard in durum wheat due to the deletion of both genes [29], which occurred during the development process in tetraploid wheat by partial removal of Ha locus from the A and B genomes [22]. | |
| Spanish Neglected and Underutilized Wheat Species | |
| Archaeological evidences have shown that wheat has been cultivated in the Iberian Peninsula, since the fifth millennium B.C. [30]. The archaeological remains found included both hulled and naked wheat. These denominations make reference to their glumes remaining intact or not, adhering to the grain after threshing. Although the term (hulled) generate some controversy, because it includes in the broad-sense, many species within the genetic complex of the wheat, as a species in the genus Haynaldia, Aegilops, or Agropyro, among others. In a narrow-sense, this denomination applies to three discrete crops: cultivated einkorn (T. monococcum L. ssp. monococcum), cultivated emmer (T. turgidum ssp. dicoccum Schrank em. Thell.), and spelt (T. aestivum ssp. spelta L. em. Thell.), which were cultivated in Spain some time ago, but currently could be catalogued as neglected or underutilized crops. Other wheat species that were cultivated in Spain in the past are: Polish wheat (T. turgidum ssp. polonicum L. em. Thell.), rivet wheat (T. turgidum L. ssp. turgidum), and club wheat (T. aestivum L. ssp. compactum Host. em. Mackey). | |
| Wheat variability in Spain was studied in the 19th century by the Spanish botanists Mariano Lagasca and Simón de Rojas Clemente in their unpublished (Ceres Hispanica) herbarium [31]. This classic study permits comparison of the variability present in the 19th century, when these crops were still widely used in Spain, to the current situation where these plants are preserved in Germplasm Banks (Table 1). | |
| However, this great diversity presently has been widely reduced to two main species: durum and bread wheat, while the rest have been lost or associated with traditional agriculture. Einkorn is considered to have practically disappeared in Spain. In fact, its use declined quickly with the introduction of tetraploid wheats being exclusively used for animal feed; although its straw has been used for making cooking utensils and in certain regions, as ceiling in huts. | |
| In Spain, emmer and spelt form a complex group called escanda (Latin: “scandÅla”), although it is possible that other less-known species are also included in the group due to their similarity with some of the species in this complex. Emmer is also called povia or pavida; whereas spelt is called Asturian fisga. However, given that emmer is practically lost, the denomination escanda could be exclusive for spelt. | |
| In Spain, both einkorn and emmer seem to have been present from the Neolithic period, although never as a main crop. Spelt, on the contrary, has been around since the Iron Age in some areas of northern Spain [30]. Later, references on its cultivation can be found around the Middle Age, both in Christian texts as the “Cronicon Albendense” dated in 883, and in Arab agronomic treatises as the “Agriculture’s Book” of Ibn Al Awam [32]. These crops were still identified by Lagasca and Rojas Clemente in the 19th century, among the collections of Triticum species included in the (Ceres Hispanica) herbarium. These authors classified four botanical varieties of einkorn, ten of emmer, and seven of spelt [31]. | |
| In the Twentieth Century, the percentage of hectares grown with hulled wheats increased with time, with an abrupt decrease during the Spanish Civil War (1936-39). After the 1930s, the popularity of hulled wheats increased until in the 1960s, when the percentages dropped quickly. Molina [33] indicated that the crop area in Asturias was ~ 1050 ha, compared to more recent data showing approximately ~ 45 ha. This decline in hectares corresponds with rural exodus, which was very important in many Spanish regions during the 60s and 70s, and mechanisation of agronomic tasks in many areas of Spain, together with the introduction of the semi dwarf wheat from International Centre for Improvement of Wheat and Maize (CIMMYT), México. The progressive disappearance of these materials was in part stopped by their inclusion in Germplasm Banks. Nowadays, spelt survives in marginal farming areas of Asturias (North of Spain), where the farmers grow it by traditional farming systems for home consumption mainly [34,35]. | |
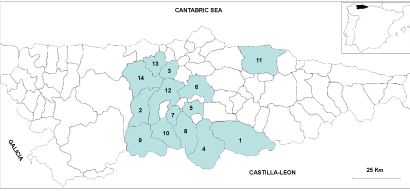
| Administratively, Asturias is divided into 78 concejos (municipalities). Escanda was cultivated in 37 of these at the beginning of the 20th century [36], with an annual production of ~ 960 tonnes. Later on in the 1930s, the staff of the Swiss Federal Research Station for Agroecology and Agriculture collected 50 populations in this region of Spain in only 23 concejos, which represents a clear reduction in the cultivation zone. Recently, one new collection mission was carried out by our group with the objective of collecting hulled wheats everywhere in this region, where these crops are still cultivated. Between the end of July and the beginning of August 2004, 32 escanda populations were found in 31 localities from 14 concejos (Figure 2). | |
| During the 2004 collection mission, we noticed the disappearance of the crop in many of the localities surveyed by the Swiss expedition of the 1930s. Only seven of these 23 concejos were common to both expeditions, which indicate a great decline in the cultivation of escanda, when compared to those indicated by Peña-Chocarro [36]. | |
| Genetic Diversity of Spanish Ancient Wheat | |
| Our group began the current research in 1999 with the aim of evaluating different types of neglected or underutilized Spanish wheats. This research focused on the species indicated in table 2. | |
| The choice of each species was based on the objectives of the current research. First, these ancient wheats were evaluated and characterized as a source of new allelic variants for enlarging the genepool of modern wheat. Second, we evaluated their potential for use in creating new products, as part of the revival in traditional foods that recently started in developed countries. | |
| Einkorn | |
| The lines we evaluated of einkorn can be grouped into four botanical varieties; three of which were not previously described in the (Ceres Hispanica) herbarium. The most abundant varieties were var. tauricum Drosd. and var. monococcum, awn colour being the only difference between both varieties, which was black for var. tauricum and white for var. monococcum. In minor frequencies were also found the var. eredvianum Zhuk., f. punctatum Stransk. and the var. nigricultum Flaksb. Both varieties showed shiny glumes, which is black for the var. nigricultum and white for the var. eredvianum [37]. | |
| The variation in seed storage proteins in Spanish einkorn was high, with three allelic variants for the Glu-Am1 locus, six for the Glu-Am3 locus, seven for the Gli-Am1 locus, and fourteen for the Gli-Am2 locus, found among the evaluated accessions. Internal variability was detected in some of these materials, which could be related to their being landraces. Up to 48 different genotypes were identified based on their origin and seed storage protein composition [38]. | |
| In einkorn, waxy protein polymorphism was very low in the current collection, so electrophoretic analyses revealed only two alleles [37], although this was larger than that found by other researchers [39-41], who did not find any polymorphism in the collections of cultivated einkorn and T. urartu. One of the alleles (Wx-Am1a) was previously described by Rodríguez-Quijano et al. [42]. The novel allele (Wx-Am1a') showed less mobility, and was only found in one accession. Both alleles showed higher mobility than the Wx-A1a allele detected in common wheat (cv. Chinese Spring). | |
| When the Pina and Pinb gene variability was evaluated in the einkorn collection, three Pina and five Pinb alleles were detected in the einkorn lines, including three novel alleles for the Pinb locus: Pinb- Am1i, Pinb-Am1j and Pinb-Am1k [41]. | |
| Emmer | |
| The most representative varieties of emmer wheat were dicoccon Körn. and tricoccum (Schübl.) Körn. These varieties, together with the majus Körn. Variety, have white glumes. The difference between both varieties (dicoccon and tricoccum) is the awn length, which is long for dicoccon and short for tricoccum. The macratherum Körn. and majus varieties were less represented, whereas the presence of the three varieties (atratum (Host.) Körn., lagascae Al. et Tell., nom. nud., and pycnurum Alef.) was very scarce [42]. Some of the varieties described by Molina and Peña [33] did not appear among the materials analysed here (var. inerme Körn., var. rufum Schübl, and var. pseudomacratherum Flaksb.), which suggests that part of the diversity of this species in Spain could have been lost in the first half of the twentieth century. | |
| When the emmer collection was evaluated for HMWGs, four allelic variants were detected for the Glu-A1 locus; one of which had not been previously described. For the Glu-B1 locus, three of the nine alleles detected had not been previously identified [43]. Similarly, a high degree of variation was found for the LMWGs, where up to 23 different patterns were detected for B-LMWGs. When considering HMWGs and LMWGs collectively, 30 combinations were found among the evaluated lines. In a later study, we selected 31 lines that showed these allelic variations and evaluated them for morphological and quality traits [42,44]. Up to seven different botanical varieties were identified, which indicated more diversity than anticipated, although lower than that for the 10 botanical varieties that had been historically described [31]. Furthermore, the allele data obtained for gluten strength also showed some of the alleles detected for HMWGs and LMWGs [44]. | |
| In contrast, emmer showed no polymorphism for the Wx-A1 locus. All the accessions evaluated (87 accessions) had the Wx-A1a allele, which could be similar to the wild allele [45]. For the Wx-B1 locus, three alleles were detected: Wx-B1b (null type) that lacks the protein; Wx-B1c*, which had the same mobility as Wx-B1c' detected in the durum wheat cultivar ‘Mexicali’, but lower electrophoretic mobility than the Wx-B1a allele, and a new allele, called Wx-B1g. This new allele showed a slightly higher mobility than the Wx-B1a allele, but this is lower than the mobility of the Wx-B1d allele found by Yamamori et al. [46-48]. The Wx-B1g allele was the most abundant, being detected in 85 accessions. The null type (Wx-B1b allele) and the Wx-B1c* allele were only detected in one accession. | |
| Because of the reduced variability for size among the different Wx alleles, the variability found was revaluated by PCR-RFLP and DNA sequence analyses [45]. These analyses showed that the Wx-A1a allele of emmer wheat is slightly different to the Wx-A1a allele of durum wheat, presenting two synonymous changes (silent mutations) in the eleventh exon that did not modify the predicted protein sequence. | |
| For the Wx-B1 gene, the molecular data confirmed three alleles, previously described by SDS-PAGE separation [45]. The deduced protein for two of them (Wx-B1b and Wx-B1g) was the same, although no protein could be detected for the Wx-B1b (null allele). Both alleles had 15 and 3 differences, respectively, with the Wx-B1a and Wx-B1c' alleles detected in the durum wheat cultivars (Langdon and Mexicali) used as standards. The other Wx-B1 allele of emmer wheat (Wx-B1c*) was also different from the standard alleles (Wx-B1a and Wx-B1c'), with 13 and one amino acid changes, respectively, and two amino acid changes with the Wx-B1b and Wx-B1g alleles. | |
| Rivet wheat | |
| The analysis of sixty accessions of Spanish rivet wheat in this collection showed high genetic diversity with sixteen botanical varieties (var. buccale Alef, var. dinurum Alef, var. dreischianum Körn, var. gentile Alef, var. herrerae Körn, var. lusitanicum Körn, var. megalopolitanum Körn, var. melanatherum Körn, var. miscibile Haçid, var. nigroglumaceum Flaks, var. nigroglumarum Haçid, var. pseudosalomonis Papad, var. rubroalbum Flaks, var. salomonis Körn, var. speciosum Alef, and var. triste-rubro-atrum Al), and five additional botanical types, according to the criteria reported by Molina and Peña [33], Additionally, five botanical types including seven lines were detected, but these could not be associated to any botanical varieties described in the (Ceres Hispanica) herbarium. | |
| Furthermore, up to 13 allelic variants (four alleles for the Glu-A1 locus and nine alleles for the Glu-B1 locus) were found in the evaluated lines, five of which were new (one for the Glu-A1 locus and four for the Glu-B1). Also, thirty-four patterns were identified in the B-LMW glutenin subunits. In all cases, the data showed low values for the effective number of alleles, as well as reduced genetic diversity, which indicated that there is a danger of genetic erosion in these loci [47]. | |
| Spelt | |
| The characterization of the spelt lines derived from the Germplasm Banks indicated that six out of the seven botanical varieties of Lagasca and Rojas Clemente [33] were present in the collection (var. albivelutinum, var. arduini, var. caeruleum, var. duhamelianun, var. rubrivelutinum and var. vulpinum); only the album variety (awnless and white glumes) was not found [48]. However, the collected populations showed certain genetic erosion with respect to the materials collected by the Swiss expedition in 1930s. Five of seven botanical varieties described by Lagasca and Rojas Clemente (var. albivelutinum, var. arduini, var. caeruleum, var. rubrivelutinum, and var. vulpinum) for spelt were detected, together with two types not mentioned by previous authors (yellow spikes with glabrous and pubescent glumes). | |
| In spelt, three allelic variants were detected for the Glu-A1 locus. For the Glu-B1 locus, two of seven alleles detected had not been found before, while four of the nine alleles detected for the Glu-D1 had not been described previously [49]. When the three loci were considered together, 25 combinations were found among the evaluated lines. For the LMWGs, extensive variation was found: 46 different patterns for the B-LMWGs and 16 for the C-LMWGs [50]. Concerning gliadins, 61 different patterns were found for the ω-gliadins, 44 for the γ-gliadins, 19 for the β-gliadins and 15 for the α-gliadins [51]. | |
| Within the spelt collection, the diversity of HMWGs was studied electrophoretically in 333 accessions, grouped in 50 populations, originally collected from Asturias (North Spain) during the 1930s. Inter and intra-population distribution of HMWGs alleles at the Glu-A1, Glu-B1 and Glu-D1 loci, together with the gliadin patterns, were investigated [51,52]. The results showed that genetic variation is mainly present within populations, with the variation between populations being only 21%. The material analysed showed polymorphism for all seed storage proteins, although some allelic variants were clearly hegemonic, while others appeared less frequently. This suggested the possibility that there was a loss of variability, even before the collection mission, which could have deteriorated further with the subsequent reduction in the cultivated area of this species in the Asturias. In fact, the materials collected during the collection mission to Asturias in 2004 [48], showed loss of nine HMWGs alleles that were detected in the old collection [49]. | |
| The electrophoretic analysis of the waxy proteins revealed polymorphism for the three waxy loci in the Spanish spelt lines that were evaluated [53]. For the Wx-A1 locus, two alleles were found: Wx- A1a, similar to the allele present in cv. Chinese Spring and Wx-A1b (null type), which lacks the protein, although subsequent studies showed that one of the Wx-A1b (null type) alleles showed low expression, being reassigned as Wx-A1g [54,55]. The sequence analysis of this allele showed the presence of a 160-bp insertion in the fourth intron that affects complete splicing of all the RNA molecules, thus reducing the yield of correctly processed RNA, leading to a significant decrease in the final concentration of Wx-A1 protein. | |
| For the Wx-B1 locus, three different alleles were found: Wx-B1a, the null protein Wx-B1b, and the Wx-B1c* allele, which showed less mobility than Wx-B1a allele. Both Wx-B1b and Wx-B1c' alleles were less common (11.67% and 12.86%) than Wx-B1a. The lines evaluated showed homogeneity for the Wx-D1 locus, where 99.52% of the accessions contained the Wx-D1a allele. Two additional alleles were detected: the null allele (Wx-D1b), which was only present in one accession, and a novel waxy allele, not previously described, with a slightly lower electrophoretic mobility than the Wx-D1a allele. This new allele was provisionally named Wx-D1g [53]. | |
| Eight combinations were detected that showed variation in the Wx-A1, Wx-B1, and Wx-D1 loci, where there was a clear dominance by the combination Wx-A1a, Wx-B1a, Wx-D1a; appearing in 69.52% of the samples. In the other loci, three combinations with a relatively high frequency were observed. The combination Wx-A1a, Wx-B1c', Wx-D1a appeared in 47 accessions, Wx-A1a, Wx-B1b, Wx-D1a in 44 accessions, and Wx-A1g, Wx-B1a, Wx-D1a in 23 accessions. The other four combinations were rare with frequencies of less than 5% [53]. | |
| Bread wheat (Mexican landraces) | |
| In 2004, a collection of one-hundred and two lines of traditional common wheat landraces (Mexican Creole wheat) grouped into 15 landraces was received. These old wheat materials are the last trace of the common wheat, carried by the Spanish to America during the 16th and 17th centuries. These materials are derived from old Spanish cultivars and landraces, and their cultivation survived on small-scale traditional farms. They could represent the nexus between the old bread wheats cultivated in Spain and the traditional varieties stored in the Germplasm Banks, and could contain genetic variability that is now missing from modern wheat. | |
| Different morphological traits were measured with the purpose of evaluating how many of the 22 botanical varieties that Lagasca and Rojas Clemente established for common wheat in Spain throughout the 19th century still existed [31]. These Mexican Creole wheats were grouped into seven botanical varieties [56]. However, because there is no data on the botanical varieties taken to the Americas during the 16th and 17th centuries, the level of genetic diversity loss in these wheats could not be determined. | |
| Up to 21 HMWG allelic variants (four, eight and nine alleles for the Glu-A1, Glu-B1 and Glu-D1 loci, respectively) were detected. The frequency distribution was very asymmetric: 90% of the lines had only two Glu-A1 alleles, 88% had two Glu-B1 alleles, and only one Glu-D1 allele was present in 81% of the lines. This suggests the possibility of erosion, mainly by genetic drift [56]. | |
| When grain hardness was measured, 16 lines were hard and 86 were soft-textured [57]. All hard lines could be explained by a mutation in either the Pina-D1 or Pinb-D1 genes. In six hard lines, there was no amplification of Pina-D1, suggesting that this gene was deleted (Pina- D1b allele). The remaining ten hard lines showed the presence of both Pina-D1 and Pinb-D1. Sequencing the Pinb-D1 genes of the hard lines revealed the presence of two different alleles (Pinb-D1b and Pinb- D1e). This latter allele has been described as rare, being found in only seven lines of bread wheat up to now, while those eight lines of Creole Mexican wheat appeared to carry the allele. Due to the origin of these wheat accessions, the presence of the rare puroindoline haplotypes may be explored in old local varieties of Spanish common wheat. | |
| In general, the results substantiate the importance of very old Mexican landraces as potential sources of genetic diversity for key quality traits in the development of modern wheat cultivars [58]. | |
| Concluding Remarks | |
| Wheat is an important crop that has been associated with human food for many centuries. It is the basis for a diverse range of products, mainly bread and pasta, and in some cases, beer, which is present in most diets worldwide. In some cases, the same wheat type has been used for all of these three different products, depending on the geographical or cultural area. For example, durum wheat was used to make bread in the Nile Valley, beer in the Euphrates Valley, and pasta in the Yangtsé Valley. In the Mediterranean region, wheat is linked to flour and its consumption. | |
| From the mid-20th century, plant breeding, based on high-yielding cultivars, has contributed to the narrowing of the genetic base of crops, causing many modern cultivars to be closely related. Subsequently, it is necessary to search for and conserve plant genetic resources to enlarge the gene pool and avoid genetic uniformity, which makes crops vulnerable to biotic and abiotic stresses. In order to face the new challenges arising due to climate change, sustainable models of agriculture would need to be adopted. Plant genetic resources can be used to enlarge the genetic background of the modern cultivars, although, in many cases, these ancient varieties are underutilized or neglected crops that could be reintroduced into agriculture. | |
| In this review, the variability of several collections of Spanish ancient wheat for three traits related to technological quality has been evaluated. In general, genetic diversity was high, although a large part of this diversity is at risk of erosion, given that the distribution of the combinations among the evaluated accessions was not random. There was an assumption that, in general, species distribution is conditioned by seed transference among farmers. This produces the homogenization of a crop or cultivar in any region after several generations, along with their irretrievable loss when these farmers stop farming the crop or cultivar. | |
| The alleles found for these loci were different to those detected in cultivated wheat. The low frequencies of the new alleles confirmed the need to protect and conserve these accessions, because it is unlikely that these alleles will be found in other crops. Therefore, the loss of these materials would be equivalent to the loss of these alleles. Although the revival of these old crops in modern agriculture is possible, it is more probable that the variation found in these species can be used by the plant-breeders for enlarging the genetic background of the modern wheat. | |
| Acknowledgments | |
| This research was supported by a grant AGL2010-19643-C02-01 from the Spanish Ministry of Economy and Competitiveness, co-financed by the European Regional Development Fund (FEDER) from the European Union. | |
| References | |
References
|
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 17983
- [From(publication date):
May-2013 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 13277
- PDF downloads : 4706
