Review Article Open Access
Special Considerations for Measuring Energy Expenditure with Doubly Labeled Water under Atypical Conditions
| Surabhi Bhutani*, Natalie Racine, Tim Shriver and Dale A Schoeller | |
| Department of Nutritional Sciences, University of Wisconsin-Madison, Wisconsin, 53706, USA | |
| Corresponding Author : | Surabhi Bhutani PhD, Department of Nutritional Sciences University of Wisconsin Madison 1415 Linden Drive, Room 407 Madison, Wisconsin 53706, USA Tel: 6082611902 Fax: 6082625860 E-mail: bhutani@nutrisci.wisc.edu |
| Received: June 29, 2015 Accepted: July 21, 2015 Published: July 30, 2015 | |
| Citation: Bhutani S, Racine N, Shriver T, Schoeller DA (2015) Special Considerations for Measuring Energy Expenditure with Doubly Labeled Water under Atypical Conditions. J Obes Weight Loss Ther S5:002. doi:10.4172/2165-7904.S5-002 | |
| Copyright: © 2015 F Bhutani S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
The global increase in the prevalence of obesity has dramatically increased interest in understanding the factors that influence human total energy expenditure (TEE). This in turn has increased interest in the doubly labeled water (DLW) method, a technique for measurement of total energy expenditure in free-living humans. The increasing use of this method is attributed to its portability, objectivity, minimal invasiveness, high accuracy and good precision. Although a relatively standard protocol for the method has emerged, the new generation of users often is unfamiliar with rationale behind aspects of the protocol as well as the approaches to avoid or correct for in situations that are not covered by the standard protocol procedure. The primary uncommon situations like introduction of isotopically different diet and fluids with or without geographical relocation, seasonal and temperature variations, activity level of participants etc. during or prior to the DLW measurements can lead to shift in baseline abundance of 2H and 18O or tracer elimination, resulting in moderate to large errors in the measured TEE. These unique situations call for special modifications to the conventional protocol to minimize errors. The objective of the present review was to assemble a list of frequently asked questions and the issues they represent, and then examine the available literature to describe and explain the modifications to the standard DLW protocol to maintain the method’s accuracy. This discussion of DLW protocol modification can be an excellent resource for investigators who intend to use this measurement technique for interesting and uncommon study designs.
If all assumptions upon which this method relies are satisfied, human energy expenditure can be measured with 2% accuracy and 2-10% precision using a standard DLW protocol [3]. The cost of enriched 18O labeled water, however, requires that loading doses of doubly labeled water be small. Because both 2H and 18O are naturally occurring, these small doses result in the natural abundances of these stable isotopes being mathematically important in the calculation of enrichments following the loading dose. Under certain study conditions, changes in physiological or environmental variables can introduce a measureable shift in the natural abundances producing errors in the calculated enrichment and hence Total Energy Expenditure (TEE). Thus, the main purpose of the present review is to (i) summarize the rationale for the DLW standard protocol, (ii) identify those unusual study situations where the accuracy of the doubly labeled water method would be impacted and (iii) discuss approaches and options to avoid or correct for such variations.
rCO2=(TBW/2)(kO - kH)
This, however, does not account for isotope fractionation or differences in isotope distribution spaces. Doing so for humans greater than 1 year of age, leads to a more complex equation [8]:
rCO2=(1/2f3)(NOkO – NDkD)-rG(f2 – f1)/2f3
Where: f is the fractionation factor for the conversion of 1) hydrogen isotopes in water vapor and liquid water, 2) oxygen isotopes in water vapor and liquid water, and 3) oxygen isotopes in CO2 and liquid water; NO and NH are the dilution spaces for labeled oxygen and hydrogen, respectively; kO and kD are the disappearance rates for labeled oxygen and hydrogen, respectively; and rG is the rate of fractionated water lost as gas (transcutaneous and lung).
Substituting for the known constants, this simplifies to [5,8]:
rCO2=0.455 TBW (1.007 kO-1.041 kH)
Where, TBW=Total Body Water in moles; kO and kH=oxygen and deuterium elimination rates in pools/day.
k=-(ln enrichmentfinal-ln enrichmentinitial)/Δt
Where, ln=natural log; enrichment=enrichment above baseline; Δt=enrichment between the initial and final sample.
Isotopic dilution space=((WA/1000a)x(da-dt)/(ds-dp))-w
Where, W=quantity of water used to make isotopic dilution in grams; a=quantity of dose water used in dilution in grams; A=quantity of dose taken by participant in grams; da=the permil isotopic enrichment measured in the diluted dose; dt=the permil isotopic enrichment measured in tap water used for dilution; ds-dp=enrichment measured in post-dose urine minus pre-dose urine sample; w=amount of water consumed between the DLW dose and 3 hour post dose urine sample.
The TBW is calculated from the average of 2H and 18O dilution space divided by 1.041 and 1.007 respectively to correct for in vivo isotopic exchange [5].
TEE(kcal/day)=22.4(3.9(rCO2/FQ)+1.1(rCO2))x4.184/1000
Where the Food Quotient (FQ) is substituted for the 24 h Respiratory Quotient (RQ), or more correctly Respiratory Exchange Ratio (RER=CO2 produced/O2 consumed). Modification may be required to account for use of storage of energy substrates, if the subject is 20% or more outside of energy balance [10].
These calculations are used to accurately measure both the total daily energy expenditure as well as body composition based on the following assumptions [3]:
1) The volume of the body water pool is constant (+/-3%) throughout the measurement.
2) With the exception of known isotopic fraction between water, water vapor and CO2, the concentrations of isotopes leaving the body are same as those in body water.
3) The isotopes label only H2O and CO2 in the body and the rapidly exchangeable H and O that equal 4.2% and 0.7% of the pool, respectively.
4) None of the two isotopic tracers that leave the body re-enters.
5) The natural abundance of both isotopes remains constant during the measurement period.
6) The average respiratory exchange ratio is equal to the food quotient for the duration of the measurement.
The most commonly used DLW protocol is the modified “two point” plateau method [7]. On the day of the DLW administration a baseline urine sample is collected to determine the enrichment and to ensure that subjects have voided before the DLW dose, followed by an overnight fasting. After the DLW oral ingestion, participants consume a small quantity (50-100 ml) of drinking water to wash out dose bottle and mouth to avoid incomplete dosing and loss of highly enriched water by evaporation from the oral cavity. Time of administration, duration of dosing and any spillage are recorded. Three additional urine samples (>20 ml to minimize artifacts due to evaporation or contamination with ambient water vapor) are collected, with the first at least 1 hour and the last two at least 3 hours post DLW dose administration [13]. These samples are then sealed and frozen until the analysis is performed. Two final urine samples are collected at the end of the period of TEE measurement, typically between 4 to 21 days depending on the turnover of the two isotopes [4]. Samples should be collected on the same time of the day (+/- 3 h) to minimize small errors due to diurnal variations in energy expenditure and water flux. Time of sample collection should also be noted correctly to make adjustments in the calculation of elimination rates. Because the time of voiding is not equal to the time the urine entered the bladder, it may add an error in the TEE measurement. However, this error is small because it occurs for every urine specimen. It is very valuable to collect and store backup samples, especially at the beginning and end of the DLW process to avoid problems related to loss or contamination of specimen.
Additional protocols are the “slope-intercept” protocol in which the urine samples are collected beginning 6 to 24 hours after the dose and the initial isotope dilution is calculated by back extrapolation of the ln (enrichment) vs. the time curve [14]. Post dose samples can be collected on day 1 and the final day of the elimination period or as frequently as daily during the elimination period. More frequent sampling can improve precision, especially if the measurement of enrichment is a major source of random error in a given laboratory, but this does increase the analytical workload [7]. Finally, the Maastrict protocol has been developed which uses an evening dosing and an overnight period before collecting the first urine to ensure isotope equilibration [15]. These protocols, although slightly different, provide very similar (+/-1-3%) results and thus decisions on which to use is typically made to best fit the practical aspects of the study.
In special populations such as infants where the equilibration period exceeds the recommended feeding schedule, food can be consumed with the dose or as soon as 0.5 hour post dose. In this instance, investigators have generally used the slope-intercept protocol because it allows calculation of the enrichment at the time of dose [14]. Among older subjects, food and beverage consumption is delayed to 1 h after dose to allow for gastric emptying of the dose, an approach that can be used with the plateau protocol [18]. Once consumed, any extra food moisture or water equilibrates with body water and therefore increases the apparent dilution space [2]. Subtracting the extra fluid intake from the isotope dilution space corrects the TBW value back to value at the time of dosing [18]. Trabulsi et al., for example, conducted a study in 484 subjects to evaluate the precision of the DLW technique for a large-scale evaluation of dietary intake instruments [12]. The researchers allowed 600 mL of liquid replacement meal (Boost, Mead Johnson Nutritionals, Evansville, IN, USA), coffee, tea, juice, or water between 1 and 3 hours of DLW. The time, type and quantity of beverage consumption was recorded to correct future TBW calculations [19]. Liquid consumption should not be allowed between 3 and 4-hour post dose unless the participant is unable to void due to slow urine production [12]. Amount of water larger than 600 mL approach quantities that have been shown to cause temporary isotopic disequilibria in the circulatory water space resulting in overestimation of the dilution spaces [20].
TBW=ND/1.042=NO/1.007
During the first year of life, these calculations may differ, suggesting that the calculation of TBW may be less prone to error [24,25].
Calculating Fat Mass (FM) and Fat Free Mass (FFM): FFM is derived from dividing the calculated TBW with the hydration coefficient of 0.732 in adults [26]. The FM can be calculated by subtracting derived FFM from body weight.
FFM(kg)=TBW/0.732
FM(kg)=body weight (kg)-FFM (kg)
The hydration values for infants, children, and adolescents vary by age [27,28].
Foods vary in abundances of 2H and 18O and will change the isotopic background when introduced during the tracer collection period. A prime example is intake of a variety of complementary foods in infants in conjunction with breast-feeding, a situation that creates a unique challenge for the DLW studies that intend to measure energy requirements of infants. Typically, a small dose of isotopic water ranging from 1 g/kg TBW- 2.6 g/kg TBW is administered to the infant and urine or saliva samples are collected between 5-15 days and has been extensively used to measure energy intake in infants who are exclusively breast-fed or formula fed [41-46]. When, however, these infants reach an age where complementary foods are introduced during the tracer collection period in conjunction with breast-feeding, the isotopic background changes. Adult studies involving a switch to a special formula diet to study the effects of specific macronutrients is another example [47]. Both are indications for utilizing strategies that can account for unknown changes in food consumption.
Correction of baseline tracer levels using an equilibration period: In situations where the dietary change to be introduced during the tracer metabolic measurement period is known in advance, it is advisable to perform an equilibration and wait for the new isotopic baseline of body water to be formed [37]. For example, Schoeller et al. measured the energy requirements of hospitalized ambulatory patients on the Total Parenteral Nutrition (TPN). Since the TPN solution is formulated using distilled or otherwise purified form of water sourced near the formulary and thus having an isotopic natural abundance different from body water, the patients were maintained on a consistent TPN diet for 10 days before administering the DLW dose [37]. This equilibration period reduced fluctuations in the natural abundance of 2H and 18O in body water due to introduction of a new water source, thus reducing errors in estimating isotopic enrichment at end of the energy expenditure measurement period. Jones et al. also performed a similar, but shorter equilibration of 4 days in infants who were put on either oral or parenteral diet post abdominal surgery [40]. Thus, an equilibration period of approximately one half-life of the body water pool is a viable option for similar study designs.
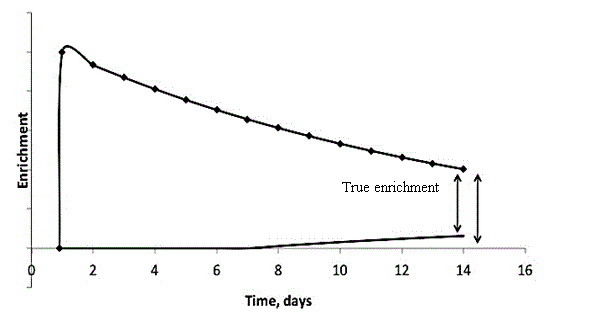
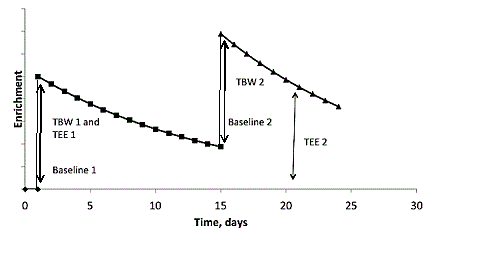
Correction of baseline tracer levels using prediction models: In cases where shift in the diet is made in the middle of the TEE measurement period, different correction methods are required to adjust to the new baseline. For instance, Jones et al. studied the precision of the DLW to measure energy expenditure in infants postoperatively, where the diet was switched from parenteral to an alternative parenteral/oral formula regimen (shift in the nutrient related water) at the midpoint of the measurement [40]. Conventionally, isotopic enrichment of the body water is measured by differences between the isotopic abundance after the DLW administration and the baseline. When the diet, and especially when the water in the diet changes post DLW dose administration, the baseline changes. That change, however, cannot be readily detected during the TEE period until the loading dose washes out in about 10 half-lives or two to three months (Figure 1). Thus, the pre-dose baseline is not a reliable representation of the true baseline. In similar situations, researchers may use the predictive equations developed by Jones et al. that take into account differences between measured initial baseline and predicted baseline on the new diet, the time interval to re-equilibration to new water, and fraction of water derived from dietary source [40]. The body water change was predicted by comparing the final isotopic abundance adjusted for normal enrichment of body fluids relative to intake, with the initial body isotopic abundance.
Obviating the error using a high loading dose: Administration of large dose of isotopes can also be an option to reduce measurement errors: Propagation of error calculations demonstrate that baseline isotopic shifts will have a negligible effect on the TEE measurement when the difference between the pre-dose baseline specimen and the predicted shifted baseline is less than 0.7% of the enrichment of the final urine specimen. In this case the error in the TEE will usually be less than 4%. While a useful approach, especially under conditions where there is little control of diet and beverage intake, this approach does increase the cost of the DLW loading dose.
Correction of the baseline isotopic shifts using an equilibration period: Introducing a period of equilibrium where subjects consume water from the new geographic location for 1-3 weeks before baseline dose administration is suggested to avoid underestimation of energy expenditure [37]. This method has been validated [48], but if the rate of turnover is low or if the subjects are traveling to a different location for a short period, this method may not be practical.
Correction of the baseline isotopic shifts using a control group: To account for the differences in isotopic proportions of water at a different location, it is also advisable to enroll a control group that does not receive any tracer. It is important that the control group should be involved in similar physical activity as the isotopic group for consistent isotopic elimination rate. The average change in the elimination rate of that control group is then used to estimate a baseline correction for the test group. Three studies used the control group data to correct for the error in raw isotope data [48-51]. DeLany et al. tested the effect of change in food and water source on energy expenditure measurement post DLW dosage administration in soldiers during a 30-day field training [48]. The control group did not receive any heavy water. A significant decrease in 2H and 18O enrichment was observed in urine samples in soldiers receiving no loading dose. These values from control soldiers were used to establish and predict the change in baseline and for calculation of the enrichment [48]. Jones et al. also studied the energy expenditure in military troops who moved to the Arctic Circle for 10-day training [51]. Similar to Delany et al., in this study control group showed a shift in the baseline isotopic enrichments by -4650 and -480 ppm per day for 2H and 18O, respectively, primarily due to low temperatures (-25 degree Celsius) and changes in food and water source. These shifts in the control group were then used to correct for the DLW measures in the experimental group [51]. In another study of energy expenditure measurement, marines were transported to a high elevation region with temperatures of -15 to 30 degree Celsius for 11-days of high intensity training exercise. The DLW was administered to all marines except the controls and corrections were made for the shifts in the isotopic baseline for the experimental group [49]. Therefore, using control subjects maintained the accuracy of DLW.
Correction of the baseline isotopic shifts using a combination of equilibration period and control group: Multiple studies measuring energy expenditure during military field missions have combined equilibration period and inclusion of control group to increase accuracy of the method. Gretebeck et al. employed both these correction methods for errors due to background isotope change, where subjects were to consume water from a given source indefinitely [53]. Eight healthy women consumed tap water enriched with 2H and 18O to resemble the water available on a typical space shuttle mission for 28-days. The same enriched water was used to prepare drinks and rehydrate food as well. Additionally, a control group was also included in the study that received the enriched water for 14-days for equilibration and given DLW on day 15. When the isotope disappearance rate was calculated without considering change in the background, an error of 2.9 MJ/day in TEE was observed in the 28-day enriched water consumption group [53]. This study validated the use of an equilibration period of minimum 2 weeks to the new water source, when the isotopic enrichment of the water source for subjects is altered. The study also confirmed the validity of a control group to track changes in the isotopic background. In cases where control group is not feasible due to limited number of subjects, baseline changes can be corrected based on the final isotope ratio of the fully equilibrated baseline isotope abundance [53]. This research group took a similar approach in another study where energy requirements were calculated for 13 healthy males during a ground based mission (control) or a space flight for 8-14 days. Levels of deuterium and 18O in the potable water for space mission are quite different from that of ground water and therefore a modified DLW method was used to compare the two groups. The enrichment levels of potable water were predicted from the hydrogen and oxygen gases used for the fuel cells and a preload of deuterium and 18O were given to each crewmember to raise their baseline isotope levels to that of potable water. Dosing before the flight changed the isotopic levels of the astronauts and reduced the differences between the isotopic abundances in preflight body water and drinking water in the flight [54]. The modified combination approach has also been successfully used in a similar study where energy expenditure was measured in soldiers during a high intensity field exercise at high altitude of Mt. Rainier by obtaining a supply of the drinking water from the destination local. Soldiers drank water from the ranger station at Mt. Rainier 10-days prior to the DLW administration bringing the body of the subjects in equilibrium with the water isotope levels in the new location. The study also employed a control group to correct for the values [50]. Similarly, the African Bush soldiers were put on a 1-week equilibration diet before high intensity field training in combination with a control group to avoid errors due to changes in the baseline body water isotopic abundances [52]. The combination method has been the most popular method to correct for baseline shifts.
Correction of the baseline isotopic shifts using a high isotopic dose: Corrections for baseline isotopic shifts can also be made by simply increasing the dose of the isotopes above the level of the baseline in the natural environment [37]. A larger dose increases the signal relative to the natural abundance of deuterium and 18O and to the random error in the isotopic measurement, thus improving the precision [53], but costs for the labeled water also increase.
Correction of the baseline isotopic shifts using a loading dose that mimics the natural isotopic abundance ratio: 2H and 18O have a covariant relationship, which means any shifts in the natural abundance of both isotopes will be in the same direction and proportional. Therefore, to dramatically reduce any errors due to change in the natural isotopic abundance with change in geographic location, it is recommended to use a loading dose that mimics the ratio of natural abundances across the world [34]. In doing so the change in baselines for the two isotopes will introduce errors into the isotope elimination rates, but those errors will cancel each other when the difference between kD and kO is taken while calculating TEE.
Delaying the loading dose: Baseline isotopic errors can occur not only when a person is transported to a remote study site, but also when a person travels large distances weeks before or during the DLW period and then returns to the dosing site. For example, if a person has traveled more than 200 miles or traveled shorter distances that involve large changes in altitude two weeks before or plans to travel during the DLW period, the baseline isotopic abundance may change [34]. To avoid errors due to changes in isotopic abundance with travel, it is recommended to delay the DLW dosing for at least one biological half-life.
Correction of errors due to seasonal variations: One way to account for errors in isotopic elimination measurement is by applying theoretical corrections for the environmental water intake via skin and lungs. For instance, Fjeld et al. measured the intake of breast milk in infants using the deuterium dose under high temperature and humidity conditions [59,60]. High water content of atmosphere and subsequent reduction in food intake resulted in non-dietary water accounting for 30% of the total water influx and therefore overestimation of milk intake. But when researchers corrected for this atmospheric water influx the error was reduced [59]. To deal with the seasonal issue, Luke et al. employed another approach in a study where energy expenditure was measured in 149 women in tropical conditions of Nigeria [61]. Due to a potential rapid body water turnover with high heat and humidity, the researchers reduced the isotope elimination measurement period in the study to 7-10 days from conventional 14-days improving the accuracy of the measurement. This resulted in higher enrichments of the tracers in the final specimens and thus reduced the influence of baseline changes.
Correction of errors by reducing the length of observation: The length of metabolic measurement period can be reduced in high activity study situations to avoid loss of precision when the final isotope enrichments become small [9]. It is important to determine the intensity of the physical activity in advance because the half-life of isotopes decreases with increased water flux and fractionation due to heavy activity. For example, Westerterp et al. administered the DLW at an interval of 7, 8 and 7 days, and recorded the isotopic elimination rates during a 3-week period of high intensity bicycle race [64]. The half-lives of isotopes were reported to be one third of the usual values in humans with normal activity levels. The short half lives were a result of high energy expenditure and researchers recommended reducing the length of the metabolic period to 2-4 days for maximum precision [64]. The same research group provided more evidence in this area by comparing the isotopic elimination rates in low activity group with a high physical activity group [22]. The daily elimination rate of 18O and 2H were two fold higher in the high activity group than the low activity group resulting in a short biological life of 4-6 days in the high activity group vs 6-10 days for low activity levels [22].
Correction of errors by correcting for fractionation: The above extreme conditions may also influence the fractionation corrections that are incorporated into the calculation of rCO2. This is because neither sweat nor urine is subject to isotope fractionation, while breath water vapor and transcutaneous water loss are [35]. Thus the proportion of water lost via fractionating routes can change during conditions that dramatically increase sweating or increase if CO2 production is increased in the absence of sweating. The potential errors are generally small (<5%), but can be minimized by using the shortened form of equation presented above which does not make an assumption based on the ratio of fractionated vs non-fractionated water loss, but rather assumes the fractioned water fluxes will be proportional to rCO2. This theoretical construct has not been tested, however. A triply labeled water method has also been proposed for correction of evaporative water loss increasing the accuracy of heavy water technique [65]. Other published equations that assume a fixed proportion will lead to small errors under these extreme conditions. To account for the errors due to isotopic fractionation of water lost on breath and transcutaneous loss (non-sweat), corrections proposed by Schoeller et al. and Fjeld et al. [8,59] were especially used by studies in soldiers and athletes [48-50,66].
The technique has also provided accurate data in studies with extreme environmental variations in temperature, altitude and humidity levels, but only when investigators make modest changes in the standard DLW protocol to minimize error secondary to baseline changes or water flux changes. The most common inaccuracies have been attributed to baseline shifts in isotopic abundance of the body, water flux and isotopic fractionation. But with careful manipulation of certain aspects of this method such as modifying isotopic dose, adjusting the length of metabolic period, correcting for fractionation and water flux and correcting for errors using information from a control group, maintaining the isotopic ratio of the dose, those inaccuracies disappeared. Therefore to avoid misuse of the technique, it is recommended that investigators should understand the basic assumptions of DLW technique, and acknowledge that the standard protocol may not be appropriate for all research designs. The need for accuracy becomes even more important when we consider the high cost of procedure and difficulties in repetition of the measurement. Hence, with careful pre-planning and slight adjustments in the protocol, the DLW technique is still considered the most accurate and a “gold standard” method for understanding aspects of energy balance.
References
- lifson N, Gordon GB, Mcclintock R (1955) Measurement of total carbon dioxide production by means of D2O18.J ApplPhysiol 7: 704-710.
- Schoeller DA, van Santen E (1982) Measurement of energy expenditure in humans by doubly labeled water method.J ApplPhysiolRespir Environ ExercPhysiol 53: 955-959.
- IHHS (2009) International Atomic Energy Agency. Assessment of body composition and total energy expenditure in humans using stable isotope techniques. Vienna.
- Schoeller DA (1988) Measurement of energy expenditure in free-living humans by using doubly labeled water.J Nutr 118: 1278-1289.
- Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, et al. (1994) Relative dilution spaces of 2H- and 18O-labeled water in humans.Am J Physiol 267: E585-590.
- Schoeller DA, Taylor PB (1987) Precision of the doubly labelled water method using the two-point calculation.Hum NutrClinNutr 41: 215-223.
- Cole TJ, Coward WA (1992) Precision and accuracy of doubly labeled water energy expenditure by multipoint and two-point methods.Am J Physiol 263: E965-973.
- Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, et al. (1986) Energy expenditure by doubly labeled water: validation in humans and proposed calculation.Am J Physiol 250: R823-830.
- WEIR JB (1949) New methods for calculating metabolic rate with special reference to protein metabolism.J Physiol 109: 1-9.
- Black AE, Prentice AM, Coward WA (1986) Use of food quotients to predict respiratory quotients for the doubly-labelled water method of measuring energy expenditure.Hum NutrClinNutr 40: 381-391.
- Schoeller DA (1983) Energy expenditure from doubly labeled water: some fundamental considerations in humans.Am J ClinNutr 38: 999-1005.
- Trabulsi J, Troiano RP, Subar AF (2003) Precision of the doubly labeled water method in a large-scale application: evaluation of a streamlined-dosing protocol in the Observing Protein and Energy Nutrition (OPEN) study. European journal of clinical nutrition 57: 1370-7.
- Wong WW, Cochran WJ, Klish WJ, Smith EO, Lee LS, et al. In vivo isotope-fractionation factors and the measurement of deuterium- and oxygen-18-dilution spaces from plasma, urine, saliva, respiratory water vapor, and carbon dioxide. The American journal of clinical nutrition 47: 1-6.
- TJ CWC (1991) The doubly labelled water method for the measurement of energy expenditure in humans: risks and benefits. . Edtion ed. In: Prentice RWaA, ed. New Techniques in Nutritional Research: London: Academic Press. 139-76 .
- Westerterp KR, Wouters L, van MarkenLichtenbelt WD (1995) The Maastricht protocol for the measurement of body composition and energy expenditure with labeled water.Obes Res 3 Suppl 1: 49-57.
- Thorsen T, Shriver T, Racine N, Richman BA, Schoeller DA (2011) Doubly labeled water analysis using cavity ring-down spectroscopy.Rapid Commun Mass Spectrom 25: 3-8.
- Berman ES, Fortson SL, Snaith SP (2012) Direct analysis of delta2H and delta18O in natural and enriched human urine using laser-based, off-axis integrated cavity output spectroscopy. Analytical chemistry 84: 9768-73.
- Schoeller DA and Jones PJH (1987) Measurement of total body water by isotope dilution: a unified approach to calculations. Edtion ed. In vivo body composition studies. London: Institute of Physical Sciences in Medicine 131-7.
- DA S (1996) Hydrometry. In Human Body Composition . Edtion ed. In: Roche A, Heymsfield SB &Lohman TG ed. Champaign: Human Kinetics pp 25-43.
- Drews D, Stein TP (1992) Effect of bolus fluid intake on energy expenditure values as determined by the doubly labeled water method.J ApplPhysiol (1985) 72: 82-86.
- Denne SC, Patel D, Kalhan SC (1990) Total body water measurement in normal and diabetic pregnancy: evidence for maternal and amniotic fluid equilibrium.Biol Neonate 57: 284-291.
- Westerterp KR, Brouns F, Saris WH, ten Hoor F (1988) Comparison of doubly labeled water with respirometry at low- and high-activity levels.J ApplPhysiol (1985) 65: 53-56.
- Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, et al. (1980) Total body water measurement in humans with 18O and 2H labeled water.Am J ClinNutr 33: 2686-2693.
- Wells JC, Ritz P, Davies PS, Coward WA (1998) Factors affecting the 2H to 18O dilution space ratio in infants.Pediatr Res 43: 467-471.
- Trowbridge FL, Graham GG, Wong WW, Mellits ED, Rabold JD, et al. (1984) Body water measurements in premature and older infants using H218O isotopic determinations.Pediatr Res 18: 524-527.
- Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, et al. (1999) Hydration of fat-free body mass: review and critique of a classic body-composition constant.Am J ClinNutr 69: 833-841.
- Hewitt MJ, Going SB, Williams DP, Lohman TG (1993) Hydration of the fat-free body mass in children and adults: implications for body composition assessment.Am J Physiol 265: E88-95.
- Wells JC, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, et al. (2010) Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y.Am J ClinNutr 91: 610-618.
- Junghans P, Derno M, Gehre M, Höfling, Kowski P, et al. (1997) Calorimetric validation of 13C bicarbonate and doubly labeled water method for determining the energy expenditure in goats.Z Ernahrungswiss 36: 268-272.
- Biondi L, D'Urso MG, Vasta V, Luciano G, Scerra M, et al. (2013) Stable isotope ratios of blood components and muscle to trace dietary changes in lambs.Animal 7: 1559-1566.
- van Gemert WG, Westerterp KR, van Acker BA (2000) Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 24: 711-8.
- Fraser I, Meier-Augenstein W, Kalin RM (2006) The role of stable isotopes in human identification: a longitudinal study into the variability of isotopic signals in human hair and nails.Rapid Commun Mass Spectrom 20: 1109-1116.
- Lifson N (1966) Theory of use of the turnover rates of body water for measuring energy and material balance.J TheorBiol 12: 46-74.
- Horvitz MA, Schoeller DA (2001) Natural abundance deuterium and 18-oxygen effects on the precision of the doubly labeled water method.Am J PhysiolEndocrinolMetab 280: E965-972.
- Schoeller DA, Leitch CA, Brown C (1986) Doubly labeled water method: in vivo oxygen and hydrogen isotope fractionation.Am J Physiol 251: R1137-1143.
- Ritz P, Cole TJ, Davies PS, Goldberg GR, Coward WA (1996) Interactions between 2H and 18O natural abundance variations and DLW measurements of energy expenditure.Am J Physiol 271: E302-308.
- Schoeller DA, Kushner RF, Jones PJ (1986) Validation of doubly labeled water for measuring energy expenditure during parenteral nutrition.Am J ClinNutr 44: 291-298.
- Jones PJ, Winthrop AL, Schoeller DA, Swyer PR, Smith J, et al. (1987) Validation of doubly labeled water for assessing energy expenditure in infants.Pediatr Res 21: 242-246.
- Roberts SB, Coward WA, Ewing G, Savage J, Cole TJ, et al. (1988) Effect of weaning on accuracy of doubly labeled water method in infants.Am J Physiol 254: R622-627.
- Jones PJ, Winthrop AL, Schoeller DA, Filler RM, Swyer PR, et al. (1988) Evaluation of doubly labeled water for measuring energy expenditure during changing nutrition.Am J ClinNutr 47: 799-804.
- Nielsen SB, Wells JC, Fewtrell MS, Eaton S, Grinham J, et al. (2013) Validation of energy requirement references for exclusively breast-fed infants.Br J Nutr 109: 2036-2043.
- Nielsen SB, Reilly JJ, Fewtrell MS, Eaton S, Grinham J, et al. (2011) Adequacy of milk intake during exclusive breastfeeding: a longitudinal study. Pediatrics 128: e907-14.
- Nielsen SB, Wells JC, Slater C, Fewtrell MS, Reilly JJ (2011) Administering labelled water to exclusively breast-fed infants in studies involving stable isotope dilution techniques. Isotopes in environmental and health studies 47: 18-25.
- Peppard RJ, Karn CA, McCabe MA, Wassall SR, Liechty EA, et al. (1993) Measurement of nutrient intake by deuterium dilution in premature infants.J Pediatr 123: 457-462.
- Salazar G, Vio F, García C, Aguirre E, Coward WA (2000) Energy requirements in Chilean infants.Arch Dis Child Fetal Neonatal Ed 83: F120-123.
- Coward WA, Sawyer MB, Whitehead RG, Prentice AM, Evans J (1979) New method for measuring milk intakes in breast-fed babies.Lancet 2: 13-14.
- Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, et al. (1996) A comparative study of different means of assessing long-term energy expenditure in humans.Am J Physiol 270: R496-504.
- DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA (1989) Field use of D2 18O to measure energy expenditure of soldiers at different energy intakes.J ApplPhysiol (1985) 67: 1922-1929.
- Hoyt RW, Jones TE, Stein TP, McAninch GW, Lieberman HR, et al. (1991) Doubly labeled water measurement of human energy expenditure during strenuous exercise.J ApplPhysiol (1985) 71: 16-22.
- Hoyt RW, Jones TE, Baker-Fulco CJ, Schoeller DA, Schoene RB, et al. (1994) Doubly labeled water measurement of human energy expenditure during exercise at high altitude.Am J Physiol 266: R966-971.
- Jones PJ, Jacobs I, Morris A, Ducharme MB (1993) Adequacy of food rations in soldiers during an arctic exercise measured by doubly labeled water.J ApplPhysiol (1985) 75: 1790-1797.
- Mudambo KS, Scrimgeour CM, Rennie MJ (1997) Adequacy of food rations in soldiers during exercise in hot, day-time conditions assessed by doubly labelled water and energy balance methods.Eur J ApplPhysiolOccupPhysiol 76: 346-351.
- Gretebeck RJ, Schoeller DA, Socki RA, Davis-Street J, Gibson EK, et al. (1997) Adaptation of the doubly labeled water method for subjects consuming isotopically enriched water.J ApplPhysiol (1985) 82: 563-570.
- Lane HW, Gretebeck RJ, Schoeller DA, Davis-Street J, Socki RA, et al. (1997) Comparison of ground-based and space flight energy expenditure and water turnover in middle-aged healthy male US astronauts.Am J ClinNutr 65: 4-12.
- Riumallo JA, Schoeller D, Barrera G, Gattas V, Uauy R (1989) Energy expenditure in underweight free-living adults: impact of energy supplementation as determined by doubly labeled water and indirect calorimetry.Am J ClinNutr 49: 239-246.
- Singh J, Prentice AM, Diaz E, Coward WA, Ashford J, et al. (1989) Energy expenditure of Gambian women during peak agricultural activity measured by the doubly-labelled water method.Br J Nutr 62: 315-329.
- Diaz E, Goldberg GR, Taylor M, Savage JM, Sellen D, et al. (1991) Effects of dietary supplementation on work performance in Gambian laborers.Am J ClinNutr 53: 803-811.
- Harbison JE, Dugas LR, Brieger W, Tayo BO, Alabi T, et al. (2015) Seasonal variation in natural abundance of 2H and 18O in urine samples from rural Nigeria.J ApplPhysiol (1985) 119: 55-60.
- Fjeld CR, Brown KH, Schoeller DA (1988) Validation of the deuterium oxide method for measuring average daily milk intake in infants.Am J ClinNutr 48: 671-679.
- Butte NF, Garza C, Smith EO, Nichols BL (1983) Evaluation of the deuterium dilution technique against the test-weighing procedure for the determination of breast milk intake.Am J ClinNutr 37: 996-1003.
- Luke A, Dugas LR, Ebersole K, Durazo-Arvizu RA, Cao G, et al. (2009) Energy expenditure does not predict weight change in either Nigerian or African American women.Am J ClinNutr 89: 169-176.
- Ruby BC, Schoeller DA, Sharkey BJ, Burks C, Tysk S (2003) Water turnover and changes in body composition during arduous wildfire suppression.Med Sci Sports Exerc 35: 1760-1765.
- Fusch C, Gfrörer W, Koch C, Thomas A, Grünert A, et al. (1996) Water turnover and body composition during long-term exposure to high altitude (4,900-7,600 m).J ApplPhysiol (1985) 80: 1118-1125.
- Westerterp KR, Saris WH, van Es M, ten Hoor F (1986) Use of the doubly labeled water technique in humans during heavy sustained exercise.J ApplPhysiol (1985) 61: 2162-2167.
- Haggarty P, McGaw BA, Franklin MF (1988) Measurement of fractionated water loss and CO2 production using triply labelled water.J TheorBiol 134: 291-308.
- Westerterp KR, Kayser B, Brouns F, Herry JP, Saris WH (1992) Energy expenditure climbing Mt. Everest.J ApplPhysiol (1985) 73: 1815-1819.
- Park J, Kazuko IT, Kim E, Kim J, Yoon J (2014) Estimating free-living human energy expenditure: Practical aspects of the doubly labeled water method and its applications.Nutr Res Pract 8: 241-248.
- Shephard RJ, Aoyagi Y (2012) Measurement of human energy expenditure, with particular reference to field studies: an historical perspective.Eur J ApplPhysiol 112: 2785-2815.
- Coward WA (1998) Contributions of the doubly labeled water method to studies of energy balance in the Third World.Am J ClinNutr 68: 962S-969S.
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 15303
- [From(publication date):
specialissue-2015 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 10611
- PDF downloads : 4692
