Case Report Open Access
Spinal Cord Lesions in Congenital Toxoplasmosis Demonstrated with Neuroimaging, Including Their Successful Treatment in an Adult
Delilah Burrowes1 Kenneth Boyer2 Charles N. Swisher3 A. Gwendolyn Noble4 Mari Sautter5 Peter Heydemann2 Peter Rabiah3 Daniel Lee5 Rima McLeod5,6,* and the Toxoplasmosis Study Group
1Department of Radiology, The University of Chicago, Chicago, IL
2Department of Pediatrics, Rush University Medical Center, Chicago, IL
3Department of Neurology, Children’s Memorial Hospital, Northwestern University, Chicago, IL
4Department of Ophthalmology, Children’s Memorial Hospital, Chicago, IL
5Department of Surgery, Division of Ophthalmology and Visual Sciences, The University of Chicago, Chicago, IL
6Departments of Pediatrics, Pathology, Committees on Immunology, Molecular Medicine, and Genetics, and the College, The University of Chicago, Chicago, IL
- *Corresponding Author:
- Rima McLeod
Department of Surgery, Division
of Ophthalmology and Visual Sciences
The University of Chicago, Chicago, IL
E-mail: rmcleod@uchicago.edu
Received Date: 5 December 2011 Revised Date: 22 December 2011 Accepted Date: 25 December 2011
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Neuroimaging studies for persons in the National Collaborative Chicago-Based Congenital Toxoplasmosis Study (NCCCTS) with symptoms and signs referable to the spinal cord were reviewed. Three infants had symptomatic spinal cord lesions, another infant a Chiari malformation, and another infant a symptomatic peri-spinal cord lipoma. One patient had an unusual history of prolonged spinal cord symptoms presenting in middle age. Neuroimaging was used to establish her diagnosis and response to treatment. This 43 year-old woman with congenital toxoplasmosis developed progressive leg spasticity, weakness, numbness, difficulty walking, and decreased visual acuity and color vision without documented re-activation of her chorioretinal disease. At 52 years of age, spinal cord lesions in locations correlating with her symptoms and optic atrophy were diagnosed with 3 Tesla MRI scan. Treatment with pyrimethamine and sulfadiazine decreased her neurologic symptoms, improved her neurologic examination, and resolved her enhancing spinal cord lesions seen on MRI.
Keywords
congenital toxoplasmosis; NCCCTS; spasticity; myelitis and optic neuritis; 3 Tesla MRI with contrast; treatment with resolution of signs and symptoms
Introduction
Congenital toxoplasmosis is a disease caused by intrauterine transmission of the parasite Toxoplasma gondii to the fetus. This occurs when a pregnant woman acquires this parasite for the first time during gestation. T. gondii is a common parasite found in approximately 1/2 of the world population [28]. The incidence of congenital infection varies. In the United States, congenital infection is estimated to occur in ∼ 1/1000–1/5000 live births. Those who present with clinical manifestations in the newborn period frequently have severe disease at birth [21]. Neurologically, this infection is characterized by calcifications, oftentimes in the basal ganglia, cortical, periventricular areas, and near the Foramen of Monro [21]. This infection can also cause hydrocephalus secondary to obstruction of the cerebral aqueduct, Foramen of Monro, or less commonly, possibly secondary to poor reabsorption of cerebrospinal fluid or destruction of brain parenchyma by the parasite [21]. To our knowledge, neither development nor worsening of spinal cord lesions in congenitally infected persons later in midlife has been reported previously. Rarely, immune-compromised persons, e.g., those with AIDS, have developed extramedullary intradural lesions or myelitis [22].
Herein, we describe the persons in our National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) cohort that have spinal cord pathology or related problems clinically and radiographically. In addition to infants, this includes an unusual patient with congenital toxoplasmosis, with no previously diagnosed immune compromise. She developed new onset of neurologic symptoms at the age of 43 years. Her symptoms progressed over 10 years with loss of visual acuity and color vision without subsequent active chorioretinitis. At the age of 53 years, she was noted to have multiple, enhancing spinal cord lesions with intramedullary involvement on MRI, using a 3 Tesla scanner. She began a course of pyrimethamine and sulfadiazine with remarkable improvement in her symptoms. With discontinuation of her anti-Toxoplasma medicines and concurrent administration of steroids for treatment of a rash due to exposure to poison oak, her symptoms recurred and progressed. Marked improvement in her clinical symptoms and neurologic signs, and myelitis documented with spinal cord MRI occurred when treatment with pyrimethamine and sulfadiazine, with leukovorin, was reinstituted. Resolution of spinal cord lesions were noted on subsequent MRI performed with contrast on a scanner capable of imaging with high resolution. Borderline intermittent hypogammaglobulmia was noted. Our patient’s clinical history is presented in detail herein, including presentation of her neuroimaging, and description of how her spinal cord lesions due to T. gondii were diagnosed and treated. This treatment resulted in clinical improvement of her symptoms of 10 years duration, and resolution of abnormalities on MRI of her cervical and thoracic spinal cord.
Materials and methods
All human studies have been reviewed by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in an appropriate version of the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study are omitted.
Diagnosis of congenital toxoplasmosis
Criteria to establish congenital T. gondii infection in the 220 persons in this cohort followed between 1981 to November, 2011 were described previously [21,28]. These include clinical findings on physical examination consistent with this diagnosis and diagnostic serologic tests or detection of the parasite.
Evaluation of and clinical findings in children
Methods of prospective, thorough and systematic evaluations at pre-specified intervals, and findings for all children in the NCCCTS cohort have been described previously [21,28]. Findings analyzed for persons considered in this present study of spinal cord lesions include detailed neurologic examination in the newborn period for those children followed from birth, and if symptoms or signs of spinal cord lesions were noted then more detailed radiographic evaluation was performed, leading to the diagnosis of 5 infants whose findings are summarized below.
Results
Spinal cord lesions and findings and outcomes in persons in the NCCCTS
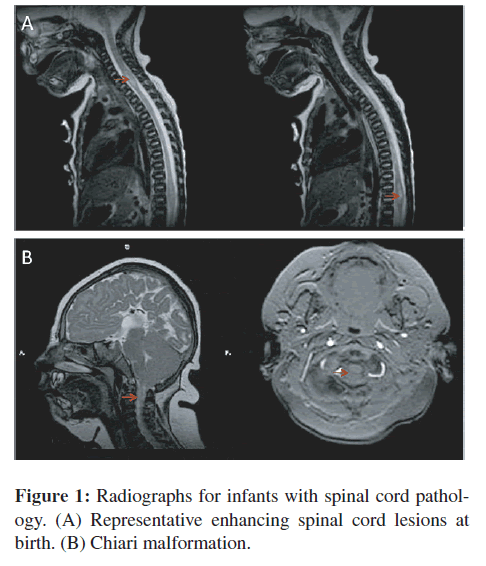
Table 1 includes spinal cord or peri-spinal cord pathologies or findings, ages at presentation of manifestations, and outcomes for six persons. Manifestations included hemiparesis, quadraparesis, upper motor neuron signs, and/or sensory levels. A representative image of a spinal cord enhancing lesion in a newborn infant is in Figure 1(A). The infant whose spinal cord is shown in Figure 1(A) had some improvement in his symptoms with treatment with pyrimethamine and sulfonamides but was left with significant deficits. Another similar infant could not be weaned from the respirator and did not survive. A third child with spinal cord lesions had significant residual deficits. A fourth child, who was described earlier, developed a spinal cord lipoma [28]. The lipoma was recognized late at one year of age although it likely developed due to perinatal treatment with steroids. This was resected after one year of age but she had residual paraparesis. Another child had a Chiari malformation with cord compression (Figure 1(B)). No other congenitally infected person other than the adult patient described in detail below, has had progressive symptoms suggestive of a spinal cord lesion in adult life, although the majority of these children have not reached 40 years of age when this patient’s symptoms began. Also most of the children in the NCCCTS cohort were treated during their first year of life and the diagnosis of the patient described in detail below had been missed when she was an infant and she had received steroids in adolescence and for short times in mid life to treat poison oak without anti-Toxoplasma therapy.
The special case of symptomatic toxoplasmic spinal cord lesions developing in mid-life with response to anti-Toxoplasma treatment
A Gravida 5, Para 4 woman delivered an apparently normal 6-pound girl at 36 weeks gestation. This child developed normally initially, apparently having normal ophthalmologic examinations.
When this child was 11 years old, she was diagnosed with congenital toxoplasmosis after she noticed gradual blurring and diminished vision in her left eye and seeing a spot in front of her left eye for two and a half weeks. She was noted to have an active 4-disc diameter lesion with a small rim of hemorrhage in her left foveal area with 20/400 visual acuity. There were also separate pigmented scars temporally and inferiorly in the left macula. She had no symptoms of her right eye with 20/25 visual acuity, but on exam she had a few vitreal cells with white scars in her right macula superiorly. There was vitreal haze in both eyes, greater on the left than on the right. Optic discs were normal bilaterally. Corresponding to her macular lesion in the right eye, her visual field showed a right paracentral scotoma. Her neurologic examination was documented to be normal at this time. Her active chorioretinitis improved with treatment with pyrimethamine, sulfadiazine, leukovorin and steroids. Unfortunately, steroids alone were continued and she experienced six episodes of recurrent eye symptoms, from this time until she was 21 years old. With each recurrent episode she was given systemic steroids for three weeks in each course. without anti-parasite medicines and she had progressive, diminishing visual acuity. The last symptomatic episode was in 1976 when she was 21 years old. She developed cataracts in each eye. The cataracts required surgery during young adulthood, at age 24 years (left eye), and at age 38 years (right eye). She was left aphakic in the left eye. She has not had refractive correction for the aphakia in the left eye presumably due to aniseikonia and poor vision in the left eye. In contrast, she is pseudophakic (posterior chamber intraocular lens) in the right eye with best corrected (−1.00+1.00 at axis 90 degrees) visual acuity improved to 20/50 one month after surgery in July 1992. The patient had a posterior capsulotomy of the left eye first in 1989 and then again in the left eye in 1993.
Multiple, cerebral calcifications were reported in skull radiographs when she was 11 years old. Her diagnosis of congenital toxoplasmosis was established by these clinical findings along with demonstration of T. gondii specific serum IgG detected with Sabin Feldman dye test (reported only as positive, no titer given), and serum direct hemagglutination test (titer 1:486). She was reported to have a skin test with toxoplasmin causing 1 cm induration, and 2 cm of redness at 48 hours with a negative control. Her mother’s serum also was positive in the Toxoplasma hemagglutination test. Serum protein electrophoresis was reported to be normal. Total immunoglobulin was 7 grams per dl and 6 grams per dl on two separate occasions.
Beginning at the age of 40 years, she noticed progressive diminution of vision without obvious active eye disease. She was determined to be “legally blind” in both eyes at the age of 47 years. Visual fields completed over the years showed central scotomas in each eye beginning in 1997. Color vision disappeared. MRI completed in 2007 and 2009 showed a narrow suprasellar optic pathway and small optic nerves suggestive of optic atrophy.
At the age of 43 years, she had the onset of difficulty walking and dragging of her right leg with progression of her symptoms during the next years. She also reported numbness and tingling of her right gluteal area, posterior upper legs, and anterior lower legs as well as in both upper arms. Cerebrospinal fluid studies were normal and there were no oligoclonal bands excluding the diagnosis of Multiple sclerosis. In March 2009, mild spasticity in her lower extremities and bilateral Babinsky signs were observed, along with a spastic gait. She favored her right leg. Sensory abnormalities were documented.
The initial MRI study on a GE Signa HDxt 3.0 T GE Systems of her spine and spinal cord was performed without contrast. This was performed on March 24, 2009. This MRI showed only degenerative disease of the cervical spine. There was no clear evidence of abnormal spinal cord signal. She was treated with pyrimethamine and sulfadiazine and her neurologic symptoms and signs improved and then resolved completely. Again, unfortunately, her anti- Toxoplasma medicines were discontinued, she received three courses of corticosteroids, with one week in tapering doses for poison oak, and her neurologic symptoms recurred. These symptoms did not improve with treatment with trimethoprim and sulfamethoxazole. She was very limited functionally by her neurologic symptoms.
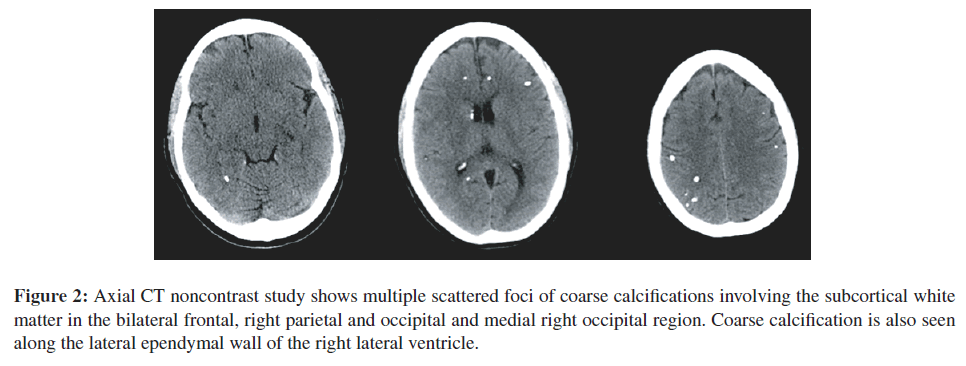
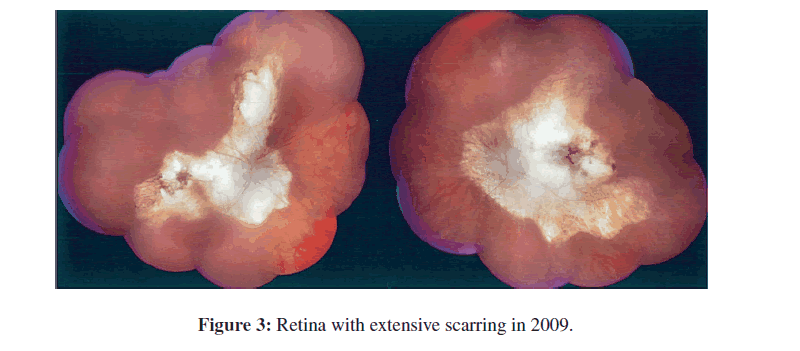
Brain CT scan completed on July 24, 2009 (Figure 2) documented her intracerebral calcifications and smaller size of one calcification in the left hippocampal area. Figure 3 shows full fundus retinal photographs in 2009.
Figure 2: Axial CT noncontrast study shows multiple scattered foci of coarse calcifications involving the subcortical white matter in the bilateral frontal, right parietal and occipital and medial right occipital region. Coarse calcification is also seen along the lateral ependymal wall of the right lateral ventricle.
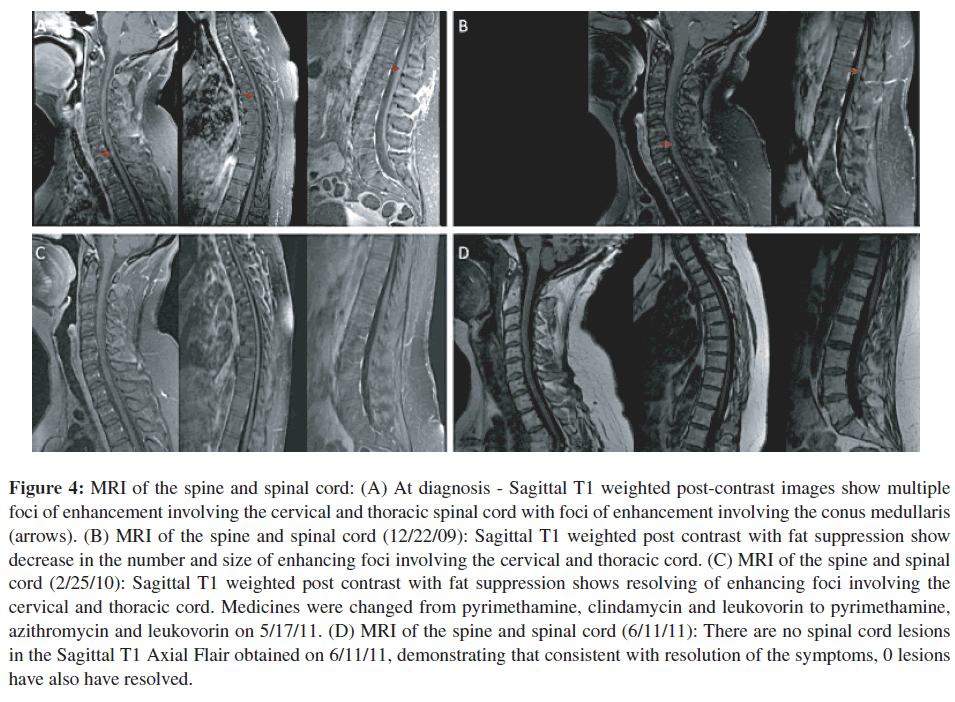
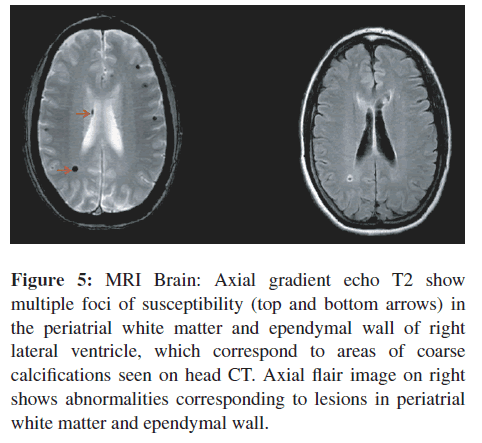
Repeat MRI examination of the spine was performed on August 28, 2009 (Figure 4(A)). It was performed with contrast on a Phillips Achieva Quasar Dual 16 3 T MRI Scanner. The images showed, unequivocally, multiple small, scattered foci of enhancement involving the cervical and thoracic cord (Figure 4(A)). These lesions were consistent with causing this patient’s symptomatology and neurologic findings. MRI images of the brain showed multiple foci of abnormal signal involving the right periatrial white matter, ependyma of the right lateral ventricle, and cortices in both right and left frontal, and parietal regions with decreased signal on gradient echo images that were consistent with intra-cerebral calcifications (Figure 5).
Figure 4: MRI of the spine and spinal cord: (A) At diagnosis - Sagittal T1 weighted post-contrast images show multiple foci of enhancement involving the cervical and thoracic spinal cord with foci of enhancement involving the conus medullaris (arrows). (B) MRI of the spine and spinal cord (12/22/09): Sagittal T1 weighted post contrast with fat suppression show decrease in the number and size of enhancing foci involving the cervical and thoracic cord. (C) MRI of the spine and spinal cord (2/25/10): Sagittal T1 weighted post contrast with fat suppression shows resolving of enhancing foci involving the cervical and thoracic cord. Medicines were changed from pyrimethamine, clindamycin and leukovorin to pyrimethamine, azithromycin and leukovorin on 5/17/11. (D) MRI of the spine and spinal cord (6/11/11): There are no spinal cord lesions in the Sagittal T1 Axial Flair obtained on 6/11/11, demonstrating that consistent with resolution of the symptoms, 0 lesions have also have resolved.
Figure 5: MRI Brain: Axial gradient echo T2 show multiple foci of susceptibility (top and bottom arrows) in the periatrial white matter and ependymal wall of right lateral ventricle, which correspond to areas of coarse calcifications seen on head CT. Axial flair image on right shows abnormalities corresponding to lesions in periatrial white matter and ependymal wall.
At this time eye examination documented with fundus photographs, visual fields and OCT indicated there has been increase in the extent of the chorioretinal scars in the posterior pole and in the periphery (Figure 3). Comparing the drawing of the chorioretinal scars present in 1987 with the examination in 2009, earlier the lesions were separate and were not contiguous in the right eye and the lesions in the left eye were contained within the vascular arcades. On examination in 2009, the lesions in the right eye are contiguous and involve a much larger area of the posterior pole, all themacula, extension superior, nasal and inferior to the optic nerve. In addition, there are peripheral lesions inferiorly. In the left eye the chorioretinal scar in the posterior pole has extended outside the vascular arcades and is also nasal to the optic nerve. In addition, there are multiple chorioretinal scars circumferentially in the periphery. The extensive, contiguous, juxtapapillary chorioretinal scar in each eye, which is mostly white with very little pigmentation, is unusual but is consistent with ocular toxoplasmosis. There is significant anisometropia (and aniseikonia) due to the pseudophakia of the right eye (−1.25+0.25 at axis 90 degrees) and the aphakia of the left eye (+10.50+1.00 at axis 90 degrees).
Treatment with pyrimethamine and sulfadiazine with leukovorin were re-initiated, and they markedly improved her neurologic signs and symptoms, with gradual recovery of normal gait. Color vision also improved slightly subjectively, although formal testing did not document improvement.
Repeat MRI’s of the spinal cord were performed on December 22, 2009, February 25 2010, and June 15, 2010 on a GE Signa HDxt 3.0 T GE Systems with contrast. These scans demonstrated improvement, diminished size and then after many months disappearance, of these foci of abnormal enhancement in the cervical and thoracic cord (Figures 4(B)–4(D)).
Immunologic evaluations showed quantitative IgG 576 mg/dl, 471 mg/dl, 533 mg/dl (normal=700−1600 mg/dl with normal subclasses). She had normal antibody response to immunization with Haemophilus Influenza Type B (HIB), normal T cell subsets, normal sedimentation rate, and normal C reactive protein level. Rheumatoid factor and cardiolipin antibody were not present. CBC with differential, liver function tests, anti-neutrophil cytoplasmic antibody = 40 (ANCA pattern), ACE level = 59 u/L (normal 7–46). There was no serum antibody to HIV in 2012 or in 1996.
She continued to take pyrimethamine and sulfadiazine with leukovorin beyond the time of resolution of her symptoms and cord lesions on MRI until July 2010. Her sulfadiazine was switched to treatment with clindamycin on 7/16/2010. Medicines were changed from pyrimethamine, clindamycin and leukovorin to pyrimethamine, azithromycin and leukovorin on 5/17/11. In a follow-up MRI, obtained on 6/11/11, there was no recurrence of her spinal cord lesions (Figure 4(D)).
Discussion
Spinal cord lesions as we have noted in patients A–C in Table 1 have been described in other reports of our cohort [reviewed in [28]]. We described patient E who had a peri-spinal cord lipoma in an earlier publication [reviewed in [28]]. We found one case report of arachnoiditis and extramedullary toxoplasmosis occurring in an immunologically normal adult with eye disease [8], a case of Guillan Barre’ complicating acute acquired toxoplasmosis in an adult [9] and congenital toxoplasmosis presenting at birth with cord involvement [28] in the literature. The adult’s history and radiographs are presented because her clinical course is unique and informative in a number of ways: she developed a treatable complication of her congenital toxoplasmosis in mid life. Spinal cord lesions were suggested by her neurologic findings but they were initially undiagnosed, and felt to be idiopathic. Ultimately they were found to be due to congenital toxoplasmosis, which was suspected by her neurologist (William Smith, MD Rochester, NY), diagnosed using a 3 Tesla MRI, and treated successfully, with efficacy of treatment documented by improvement of her neurologic symptoms and with MRI. There are few persons with untreated congenital toxoplasmosis followed into middle age whose clinical course has been described. To our knowledge, congenital toxoplasmosis has not been recognized before to cause late presenting symptomatic spinal cord lesions in mid-life. The presence of spinal cord lesions in a congenitally infected person, without known immune compromise, causing new symptoms when she was middle aged apparently is uncommon. To have spinal cord lesions, and progressive optic neuritis due to Toxoplasma gondii, causing symptoms, demonstrable on MRI, and responsive to treatment with pyrimethamine and sulfadiazine has not to our knowledge been reported previously. Most remarkable is the resolution of neurologic symptoms and signs on MRI with treatment of her toxoplasmosis with pyrimethamine and sulfadiazine.
| Patient | Spinal cord or peri-spinal cord pathologyor finding | Age at presentationof manifestation | Outcome |
|---|---|---|---|
| A | Enhancing spinal cord lesion due to T. gondii | Birth | Died in perinatal period |
| B | Enhancing spinal cord lesion due to T. gondii | Birth | Partial response in signs to anti-Toxoplasma |
| C | Spinal cord lesion due to T. gondii | Birth | medicines but significant residual deficitPersistent quadriplegia |
| D | Chiari malformation | Birth | Persistent signs and symptoms |
| E | Peri-spinal cord lipoma | Diagnosed at 1-year | Resection of lipoma when recognized at 3 yearsof age without resolution of symptoms and signs |
| F | Spinal cord lesions secondary to T. gondii. | 43 years old | Symptoms, signs, and MRI improved with Rx. |
Table 1: Manifestation and outcomes of spinal cord abnormalities in NCCCTS persons.
All the infants with spinal cord lesions in the NCCCTS had profoundly severe congenital toxoplasmosis diagnosed at birth. The congenitally infected adult described herein, although born prematurely, otherwise was reported to be well in the newborn period and was not diagnosed until her eye disease reactivated when she was eleven years old. The late development of spinal cord lesions in an otherwise healthy congenitally infected adult when he/she is in her fifth decade and documentation and responsiveness of her T. gondii cord lesion to anti-Toxoplasma medicines appears not to have been noted before.
Meningitis and encephalitis have been reported in adults with postnatally acquired T. gondii infection [31]. They had diffuse encephalitis and dural enhancement as presentation. In the literature there is a case report of chronic spinal arachnoiditis in an adult patient with HIV infection [22]. There was histologic documentation post mortem of spinal cord toxoplasmosis corresponding to involvement seen on MRIs.
Our patient demonstrated foci of enhancement within the spinal cord at the time of her clinical presentation. In general, abnormalities that involve the spinal cord typically present with T2 signal changes and the differential diagnosis for these findings includes demyelinating processes, which were excluded for our patient. The lack of associated T2 signal changes is also an unusual feature that might have been related to technique. Follow up studies, however, no longer showed these abnormal enhancing foci, which corresponded to the patient’s clinical improvement. Demonstration of the spinal cord lesions required use of 3 Tesla magnet which has higher resolution than the lower strength magnets and is the modality of choice for evaluating abnormalities involving the spine, particularly spinal cord abnormalities.
This type of presentation, but with much more fulminant symptoms, is also a feature of this infection in immune compromised persons such as those undergoing stem cell transplantation or with HIV infection. This suggests that the parasite may reach the spinal cord as it disseminates and can recur there as well.
It is not likely that our patient’s intermittent borderline hypogammaglobulinemia contributed to this patient’s neurologic disease since she responded robustly to immunization with HIB. B cells and IgM can help protection against this infection [4] but it is almost always deficits in cell-mediated immunity that are associated with reactivation of toxoplasmosis. This patient did have intermittently low IgG but there were no associated abnormalities or cell mediated immunity documented that were diagnostic of specific immune deficiency.
Progressive loss of visual acuity with loss of color vision and small optic nerves on MRI suggest that optic neuritis had also occurred in conjunction with development of her spinal cord lesions. There has been no significant, objectively documented improvement in her color vision with treatment, although she reports minimal subjective improvement.
Remarkably and importantly, radiologic studies facilitated making this diagnosis objectively. Her subjective improvement, and separate documented improvement in diminished signs correlated with resolution of her neuroimaging findings; this occurred despite the long time she had symptoms prior to initiation of treatment. This case report demonstrates that both late ophthalmologic and neurologic signs and symptoms can occur in those with congenital toxoplasmosis, including spinal cord lesions, and that these symptoms and signs can respond to medical treatment if Toxoplasma gondii is diagnosed as the cause and the lesions are treated.
Review of the literature [1-33] shows that virtually all cases of toxoplasmic myelitis or spinal cord lesions occurred either in persons who were immunecompromised by HIV infections or malignancies or their treatments or occasionally in infants with congenital toxoplasmosis at birth [3,23,28]. Herein, we describe our experience in the US National Collaborative Congenital Toxoplasmosis Study, where we have encountered 3 newborn infants with toxoplasmic spinal cord lesions. Each of these infants had spinal cord lesions that resulted in motor dysfunction. One child developed a paraspinous lipoma associated with steroid use in the newborn period. There are 214 other congenitally infected persons in our NCCCTS cohorts. Nonetheless, there have been no other congenitally infected persons in our cohorts who developed symptomatic spinal cord lesions in mid-life as adults to date although most of these patients are much younger than the woman described herein. Our patients’ symptoms might have been due to other neurologic processes such as multiple sclerosis which were excluded. Their etiology was not determined for 10 years as they progressed. Another aspect of the present case that is unique is the diagnosis and documentation of toxoplasmic spinal cord lesions for this immunologically normal middle aged woman using the 3 Tesla magnet. Also documentation of her clinical response and robust response to treatment documented with neuroimaging has not previously been reported to our knowledge. Symptomatology in a congenitally infected person developing in mid-life, as reported herein, provides evidence of a presentation of this infection, which could be confused with multiple sclerosis or other neurologic diseases. The robust response to treatment clinically and radiographically is another key finding with potential utility and benefit for others similarly affected in the future.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R01 AI027530. We would like to thank Dr. Anthony Sorge, Dr. Mark Shelly, Dr. Harold Lesser, and Dr. David Smith for their insights, and collaborations in the care of this patient. Dr. Smith first recognized and diagnosed the etiology of this patient’s neurologic symptoms and initiated her first course of treatment for her toxoplasmic myelitis. We would like to thank the patients and families of the NCCCTS for their generosity and cooperation in working with us. Other members of the Toxoplasmosis Study Group include Michael Kohrman, Shawn Withers, Theodore Karrison, Kristen Wroblewski, Paul Meier, Ronald Thisted, Jack Remington, David McClone, Dianna Bardo, Audrey Cameron, Ellen Holfels, Paul Latkany, Douglas Mack, John Marcinak, James McAuley, Marilyn Mets, Sanford Meyers, William Mieler, Dushyant Patel, Jeanne Perkins, James Rago, Nancy Roizen, Lazlo Stein, Andrew Suth,MarieWeissbourd, Teri Hull, Israel Gross, Kathy Zebracki, and Caitlin Roache.
References
- S. al Shahwan, M. L. Rossi, and M. A. al Thagafi, Ascending paralysis due to myelitis in a newborn with congenital toxoplasmosis, J Neurol Sci, 139 (1996), 156–159.
- S. Arshad, D. Skiest, and E. V. Granowitz, Subacute onset of paralysis in a person with AIDS, AIDS Read, 19 (2009), 32–35.
- L. Barraquer-Bordas and A. Muinos, Ocular pathology and spinal syndrome of chronic evolution possibly secondary to toxoplasmosis, Rev Esp Otoneurooftalmol Neurocir, 21 (1962), 219–222.
- V. Brinkmann, S. D. Sharma, and J. S. Remington, Different regulation of the L3T4-T cell subset by B cells in different mouse strains bearing the H-2k haplotype, J Immunol, 137 (1986), 2991–2997.
- H. Budka, Neuropathology of myelitis, myelopathy, and spinal infections in AIDS, Neuroimaging Clin N Am, 7 (1997), 639– 650.
- C. Campo, V. Navarro, J. A. Mota, J. Lacruz, and M. Santos, Spinal cord lesion in a patient with human immunodeficiency virus infection, Enferm Infecc Microbiol Clin, 19 (2001), 31–33.
- M. Carteret, E. Petit, O. Granat, M. Marichez, and J. Gilquin, Spinal cord toxoplasmosis and AIDS, J Radiol, 76 (1995), 453– 455.
- T. E. Cosan, S. Kabukcuo�?�?glu, A. Arslantas, M. A. Atasoy, N. Dogan, I. Ozgunes, et al., Spinal toxoplasmic arachnoiditis associated with osteoid formation: a rare presentation of toxoplasmosis, Spine, 26 (2001), 1726–1728.
- J. Couvreur and P. Thulliez, Acquired toxoplasmosis of ocular or neurologic site: 49 cases, Presse Med, 25 (1996), 438–442.
- F. Dubois, H. Petit, O. Godefroy, C. Bonte, and J. D. Guieu, Acute spinal amyotrophy in toxoplasmosis, Rev Neurol (Paris), 145 (1989), 857–859.
- C. Garcia-Gubern, C. R. Fuentes, L. Colon-Rolon, and D.Masvidal, Spinal cord toxoplasmosis as an unusual presentation of AIDS: case report and review of the literature, Int J Emerg Med, 3 (2010), 439–442.
- C. Geny, R. Gherardi, P. Boudes, F. Lionnet, P. Cesaro, and F. Gray, Multifocal multinucleated giant cell myelitis in an AIDS patient, Neuropathol Appl Neurobiol, 17 (1991), 157–162.
- M. Guti´errez Molina, C. Morales Bastos, F. Jim´enez S´anchez, P. Gonz´alez Peramato, and M. P. Su´arez Mier, Neuropathology of HIV infection, Arch Neurobiol (Madr), 52 (1989), 30–44.
- T. M. Harris, R. R. Smith, J. R. Bognanno, and M. K. Edwards, Toxoplasmic myelitis in AIDS: gadolinium-enhanced MR, J Comput Assist Tomogr, 14 (1990), 809–811.
- D. H´enin, T. W. Smith, U. De Girolami, M. Sughayer, and J.- J. Hauw, Neuropathology of the spinal cord in the acquired immunodeficiency syndrome, Hum Pathol, 23 (1992), 1106– 1114.
- S. Herskovitz, S. E. Siegel, A. T. Schneider, S. J. Nelson, J. T. Goodrich, and G. Lantos, Spinal cord toxoplasmosis in AIDS, Neurology, 39 (1989), 1552–1553.
- C. S. Koh, N. Tsukada, N. Yanagisawa, S. Yajima, and H. Tsukagoshi, A case acute acquired toxoplasmosis with acute transverse myelopathy (author’s transl), Rinsho Shinkeigaku, 21 (1981), 158–164.
- D. H. Kung, E. A. Hubenthal, J. Y. Kwan, S. A. Shelburne, J. C. Goodman, and J. S. Kass, Toxoplasmosis myelopathy and myopathy in an AIDS patient: a case of immune reconstitution inflammatory syndrome?, Neurologist, 17 (2011), 49–51.
- A. Lle´o, M. Planella, and P. Domingo, Myelopathy and human immunodeficiency virus infection, Med Clin, 112 (1999), 423– 427.
- E. Maciel, I. Siqueira, A. C. Queiroz, and A. Melo, Toxoplasma gondii myelitis in a patient with adult T-cell leukemia-lymphoma, Arq Neuropsiquiatr, 58 (2000), 1107–1109.
- R. McLeod, K. Boyer, T. Karrison, K. Kasza, C. Swisher, N. Roizen, et al., Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study, Clin Infect Dis, 42 (2006), 1383–1394.
- M. Mehren, P. J. Burns, F. Mamani, C. S. Levy, and R. Laureno, Toxoplasmic myelitis mimicking intramedullary spinal cord tumor, Neurology, 38 (1988), 1648–1650.
- M. Mehren, P. J. Burns, F. Mamani, C. S. Levy, and R. Laureno, Toxoplasmic myelitis mimicking intramedullary spinal cord tumor, Neurology, 38 (1988), 1648–1650.
- S. Nag and A. C. Jackson, Myelopathy: an unusual presentation of toxoplasmosis, Can J Neurol Sci, 16 (1989), 422–425.
- B. Ostertun, W. Dewes, H. S¨uuss, A. Steudel, H. Brassel, and T. Harder, MR tomography of non-tumor diseases of the brain and cervical cord, Rofo, 148 (1988), 408–414.
- Y. Pittner, J. F. Dufour, G. David, A. Boibieux, and D. Peyramond, Spinal cord toxoplasmosis in HIV infection, Med Mal Infect, 39 (2009), 401–405.
- T. P. Poon, V. Tchertkoff, G. F. Pares, A. V.Masangkay, M. Daras, and J.Marc, Spinal cord toxoplasma lesion in AIDS:MR findings, J Comput Assist Tomogr, 16 (1992), 817–819.
- J. Remington and J. Klein, eds., Infectious Diseases of the Fetus and Newborn Infant, Elsevier Saunders, Philadelphia, PA, 2011, pp. 947–1091.
- D. K. Resnick, C. H. Comey, W. C. Welch, A. J. Martinez, W. W. Hoover, and G. B. Jacobs, Isolated toxoplasmosis of the thoracic spinal cord in a patient with acquired immunodeficiency syndrome. Case report, J Neurosurg, 82 (1995), 493–496.
- C. S. Straathof, L.M. Kortbeek, H. Roerdink, P. A. Sillevis Smitt, and M. J. van den Bent, A solitary spinal cord toxoplasma lesion after peripheral stem-cell transplantation, J Neurol, 248 (2001), 814–815.
- J. J. Townsend, J. S. Wolinsky, J. R. Baringer, and P. C. Johnson, Acquired toxoplasmosis. A neglected cause of treatable nervous system disease, Arch Neurol, 32 (1975), 335–343.
- R. Vyas and J. R. Ebright, Toxoplasmosis of the spinal cord in a patient with AIDS: case report and review, Clin Infect Dis, 23 (1996), 1061–1065.
- R. Wende-Fischer, C. Ehrenheim, R. Heyer, M. Rittierodt, and J. H. Ehrich, In spinal symptoms remember toxoplasmosis, Monatsschr Kinderheilkd, 141 (1993), 789–791.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 13387
- [From(publication date):
December-2012 - Sep 20, 2024] - Breakdown by view type
- HTML page views : 9061
- PDF downloads : 4326