Research Article Open Access
Synthesis of Dox Drug Conjugation and Citric Acid Stabilized Superparamagnetic Iron-Oxide Nanoparticles for Drug Delivery
| Pramod Kumar*, Shrish Agnihotri and Indrajit Roy | |
| Department of Chemistry, University of Delhi, Delhi-110007, India | |
| Corresponding Author : | Pramod Kumar Department of Chemistry, University of Delhi, Delhi-110007, India Tel: +91-9711065966 E-mail: pramodgang03@gmail.com |
| Received: December 10, 2015; Accepted: January 06, 2016; Published: January 13, 2016 | |
| Citation: Kumar P, Agnihotri S, Roy I (2016) Synthesis of Dox Drug Conjugation and Citric Acid Stabilized Superparamagnetic Iron-Oxide Nanoparticles for Drug Delivery. Biochem Physiol 5:194. doi:10.4172/2168-9652.1000194 | |
| Copyright: © 2016 Kumar P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
In this manuscript, we report a novel, low cost and easy synthesis, iron-oxide nanoparticles were synthesized via room-temperature reduction of a mixture of ferric and ferrous salts, containing citric acid as capping agent. Next, anticancer drug doxorubicin (Dox) was used to form electrostatic conjugation with these nanoparticles. The resulting drug-nanoconjugates were characterized for their size, composition, functionality, crystallinity, along with their magnetic and optical behavior. Following that, they were treated with cultured lung carcinoma cell lines (A 549) to probe their non-toxicity and biocompatibility. Concurrently, their uptake in cells in culture was studied by optical bioimaging. In vitro studies have shown that these nanoparticles are nontoxic (using MTT assay) to cells in culture.
| Keywords |
| Fe3O4; DOX; Dox-CA-SPION; VSM |
| Introduction |
| One of the major challenges in Nanomedicine is targeted delivery of drugs to target sites of interest that is to increase the concentration of drug on desired sites relative to others and to enhance the interaction of drug to diseased tissues rather than healthy tissues. Drugs in their free form, once administered systemically in the body, usually diffuse to different parts of the body. As a result, not only very little drug reaches the intended site of action (e.g. Diseased site), but also causes unwanted side effects in non-target organs. Therefore, it is critically important to devise ways to concentrate the drug at the diseased site for improved therapeutic outcome. Nanoparticle based drug delivery can reduce this problem partially by using receptormediated drug delivery, whereby biorecognition agents (ligands) attached with drug-nanoparticle conjugates can guide the drugs to targeted sites [1-4]. However, the preparation of such targeted nanoformulations in optimal proportions is difficult, and often results in their uncontrolled size increase. Furthermore, these targeted nanoformulations may become immunogenic as most biorecognition ligands are protein/peptide based. An alternative way of nanoparticle-mediated targeted drug delivery involves transport the drug-nanoparticle conjugates to target sites using external stimulus, such as light and magnetic field [5-8]. Magnetic field assisted drug delivery is highly promising as it involves the use of nonionizing radiation with no restriction on their penetration depth across biological tissues. Although this mode of drug delivery was restricted to already-identified and localized diseased sites, it nevertheless offers great promise in side-effect free treatment of diagnosed solid tumors and other malignancies. |
| Iron-oxide nanoparticles are extremely promising for medical applications owing to their ultra-small size, superparamagnetic properties, ease of synthesis, and biocompatibility [9-12]. Their most popular application is contrast enhancement in magnetic resonance imaging (MRI) [13-16]. Moreover, their ability to readily respond to external magnetic field has led to several promising applications, such as magnetic field-directed cell separation, triggering of local hyperthermia for localized anticancer activity, and magnetically-guided drug delivery [17,18]. Magnetic field assisted delivery of active molecules by ironoxide nanoparticles can lead to several medical benefits, which include targeted delivery of chemotherapeutic agents for combination of MRI diagnostics and chemotherapy (theranostics), combination with optical agents for combined MR and optical imaging, etc. [19-22]. |
| In this work, iron-oxide nanoparticles were synthesized via room-temperature reduction of a mixture of ferric and ferrous salts, containing citric acid as capping agent. Further, anticancer drug doxorubicin (Dox) was used to form conjugates with these nanoparticles. The resulting drug-nanoconjugates were characterized for their size, composition, functionality, crystallinity, along with their magnetic and optical behavior. Following that, they were treated with cultured lung carcinoma cell lines (A-549) in vitro, as well as whole blood, to probe their non-toxicity and biocompatibility. Concurrently, their uptake in cells in culture was studied, with and without magnetic guidance. Finally, the effect of drug-conjugated nanoparticles to cause drug-induced cytotoxicity in cells in vitro was investigated, with and without magnetic guidance. |
| Experimental Section |
| Materials |
| Ferric chloride (FeCl3.6H2O), ferrous sulphate (FeSO4.6H2O), ammonia solution (25%), was purchased from Merck. Doxorubicin hydrochloride (Dox), 2,9-dimethyl (1,10-phenanthroline), 3-(4,5-dimethylthiazol-2-yl), 5-diphenyltetrazolium bromide (MTT reagent), fetal bovine serum (FBS), phosphate buffer saline (PBS), Dulbecco’s modified eagle’s medium (DMEM), penicillin/streptomycin, and amphotericin-B were purchased from Sigma-Aldrich. Citric acid monohydrate (CA) was purchased from Spectrochem Pvt. Ltd. (Mumbai, India). Lung carcinoma cell lines (A-549) were purchased from ATCC, VA, and cultured according to instructions supplied by the vendor. Unless otherwise mentioned, cell culture products were obtained from Thermo Fisher scientific. All chemicals were used without any further purification. |
| Synthesis of citric acid stabilized superparamagnetic iron oxide nanoparticles (CA-SPION) |
| Synthesis of citric acid stabilized superparamagnetic iron-oxide nanoparticles (CA-SPIONs) at room temperature has been carried out using the chemical precipitation method [23,24]. The aqueous solution of 2 mmol of FeCl3.6H2O, 1 mmol of FeSO4.6H2O, and 1 mL of HCl solution (50 μL 12 N HCl diluted to 1 mL by DDW) was mixed with 40 mL N2 gas-purged double distilled water (DDW). Synthesis of the nanoparticles was accomplished by the addition of iron salt solution to 5 mL aqueous ammonia solution (25%) drop-wise with vigorous stirring for 30 seconds, resulting in dark suspension of SPION. After that the stabilizer solution, i.e., 1 mL aqueous (0.5 mg/mL) citric acid, was added and further stirring carried out for 30 minutes at room temperature. The resulting CA-SPION mixture was purified via centrifugation (10,000 rpm, 10 minutes) and washed with 2-3 times with ethanol and finally resuspended in water just by sonication for 10 minutes at bath sonicator. |
| Formation of electrostatic conjugates of doxorubicin with CA-SPION (Dox-CA-SPION) |
| The Dox-CA-SPION conjugates were formed by reaction of CASPION with excess Dox in aqueous phase. The free carboxylic acid groups on the surface of CA-SPION electrostatically-reacted with the amino groups present in Dox. Here, 50 μL aqueous dispersion of CASPION (2 mg/mL) was added to a 1 mL of Dox solution (10 mg/mL in DMSO), and mixed with shaking at room temperature for 2 minutes [25]. After formation of the drug-nanoparticle conjugates, they were separated from the free drugs via magnetic separation and washing in the aqueous medium. |
| Characterization Studies |
| The magnetic properties of the dried nanoparticles were probed using vibrating sample magnetometer (VSM), using a Model 3463- 60 Electromagnet Amplifier (CREST Performance CPX 900 power amplifier Instrument). The sizes of the CA-SPIONs, with and without Dox conjugation, were determined using transmission electron microscopy (TEM). For TEM, the aqueous dispersions were sonicated and dropped-coated and dried onto formvar coated 200 mesh, copper grids (Ted Pella, USA), followed by imaging using a TECNAI G2- 30 U TWIN TEM instrument (FEI, Eindhoven, The Netherlands) with an acceleration voltage of 300 KV. The same instrument was used for probing the elemental composition (using energy dispersive spectroscopy, or EDX) and crystalline diffraction pattern (using selected area electron diffraction, or SAED) of the nanoparticles. The Size of the nanoparticles was further analyzed by dynamic light scattering (DLS) measurements, using a NANO-ZS series MALVERN ZETASIZER instrument. Helium-Ne laser (wavelength 633 nm, power 4 mW) was used as the light source. The same instrument was used to measure the surface charge (zeta potential) of the nanoparticles. High resolution powder x-ray diffraction (XRD) was used to analyze the phase composition of the nanoparticles, using a Brukar D8 Discover x-ray spectrometer, over the 2θ range from 20º–65º at a rate of 2.58/ min, using Cu-Kα radiation (λ=1.54060 Å). The UV-visible absorption and fluorescence emission measurements of the various aqueous nanoparticulate samples, both with and without conjugated Dox, were recorded using a Shimadzu UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan) and a Cary Eclipse fluorescence spectrometer (Varian, Palo Alto, CA), respectively. FTIR spectra (resolution: 4 cm-1) were acquired using a Perkin Elimer RX1 spectrometer, where 2 mg of dried and powdered nanoparticles were mixed with KBr and pressed into a pellet for analysis. The spectrum was taken from 4000 to 400 cm-1. NMR Spectra were taken using a 400-MHz spectrometer (JNM-ECX- 400P; JEOL, Tokyo, Japan). |
| Determination of drug loading efficiency |
| For determining the drug loading efficiency, the Dox-CA-SPION conjugates were first separated from free Dox in solution via magnetic separation. The fluorescence spectra of the supernatant obtained after magnetic separation of free Dox from Dox-CA-SPION) were then recorded using Cary Eclipse fluorescence spectrophotometer. The fluorescence spectra of 1 mL of pure Dox solution (10 mg/mL) were also taken. For comparison the fluorescence spectrum of initial amount of pure Dox aqueous solution (50 μL of 1.72 Mm Dox conc.=85 μM). The fluorescence intensities of supernatants (washed drug molecules were also taken into consideration for calculations) against pure Dox solution were used to determine the loading efficiency. |
| The loading efficiency (w/w %) was calculated using the following relation: |
| Drug loading (%) = [(Weight of drug in NPs) / (Weight of drug in NPs)] × 100 |
| Drug entrapment efficiency (%) = [(Weight of drug in NPs) / ( Weight of drug in NPs)] × 100 |
| Drug-release studies |
| We have investigated the time-dependent release behavior of Dox from Dox-CA-SPION by dialyzing them in a solution containing PBS for 70 hours. As owing to the low cutoff (12-14 KDa) pore size of the dialysis membrane only free Dox molecules can come out of the membrane, we have estimated the amount of released Dox by measuring the Dox concentration (via fluorescence spectrometry) in the bulk solution as a function of dialysis time. |
| In vitro studies |
| We first probed the potential toxicity of these nanoparticles (drugfree) in the blood, using hemolysis assay. After that, we carried out studies probing the interaction of these nanoparticles, with and without linking drug, using A-549 lung cancer cells in vitro. These cells were maintained in DMEM medium with 10% FBS and 1% antibiotics (Penicillin and Streptomycin), and cultured according to manufacturer’s instructions. |
| Analysis of potential in-vitro cytotoxicity of SPION and CASPION |
| We then investigated the potential non-specific cytotoxicity of non-drug conjugated SPIONs and CA-SPIONs in A-549 cells using cell viability (MTS) assay. One day prior to treatment, the cells were trypsinized and resuspended in fresh media. Then, about 25,000 cells/0.5 mL media were added to each well of a sterilized 24-well plate, and transferred back to the incubator for attachment and overnight growth. Next day, to the cells at a confluence of 30-40%, three different dosages of SPIONs and CA-SPIONs were added, mixed by swirling, and transferred back to the incubator. Control cells received PBS treatment only. After 48 hours of incubation, the plate containing the cells was taken out, the cells in each well were washed 3 times with sterile PBS, and treated with 100 μL of MTS reagent [3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide, (5 mg/mL in PBS)] for 2 hours. The resulting blue-colored formazan crystals were dissolved in DMSO, and their optical densities were recorded at 570 nm using UV–visible spectrophotometry. The optical density of each solution reflected the viability of the cells in each well. The percentage viability of the treated cells was calculated after comparing their optical density with that of PBS treated cells (positive control), the latter being arbitrarily assigned 100% viability [26,27]. |
| Monitoring of cellular uptake of drug-doped (Dox-CASPION) nanoparticles in vitro via fluorescence microscopy |
| After probing the potential toxicity of non-drug linked nanoparticles in the blood and A-549 cells, we began our analysis of interaction of druglinked nanoparticles (Dox-CA-SPIONs) with A-549 cells in vitro. Here, first we monitored the cellular uptake of the drug-linked nanoparticles. One day prior to treatment, the A-549 lung cancer cells were seeded in sterilized 6-well plates (2,00,000 cells/ 2 mL media in each well) and kept in the incubator. Next day, to the cells at a confluence of 60–70%, 2 mg/ml of Dox-CA-SPION sample were added, mixed by swirling, and kept in the incubator for 2 hours. After incubation, the plate was taken out and the cells in each well washed twice with sterile PBS, and fixed by a mixture of methanol and glycerol. The fixed cells were visualized under a fluorescent Nikon TS-100 inverted microscope, and photographed using a Nikon DIGITAL SIGHT DS-Fi1 Camera (Nikon, Japan). The fluorescence of Dox observed from the cells qualitatively correlated with the nanoparticle uptake. |
| Results and Discussion |
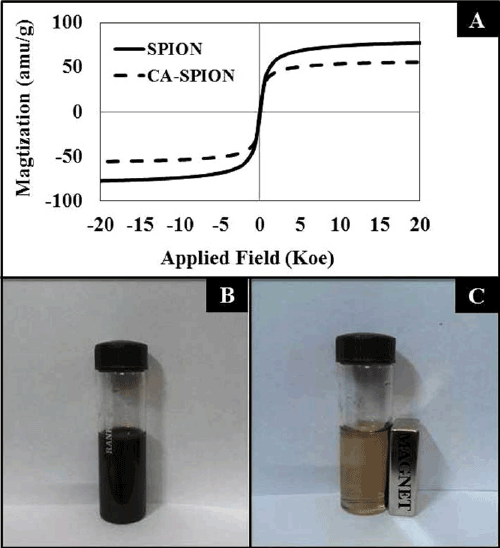
| Figure 1A shows the magnetization curves of the superparamagnetic iron-oxide nanoparticles, without (SPION) and with (CA-SPION) coated with citric acid. These curves were recorded at room temperature using vibrating sample magnetometer (VSM). Under a large external field, the magnetization of the particles aligns with the field direction and reaches its saturation value for both these nanoparticles. This pattern is indicative of superparamagnetic nanoparticles [26]. The saturation magnetization (Ms) values of SPION and CA-SPION were 68.0 and 58.0 emu/g, respectively. This result shows that citric acid coating did not significantly alter the magnetic behavior of SPION. Figure 1B shows the colloidal stability of aqueous dispersed CA-SPION, which does not have a tendency to precipitate and settle down. However, these nanoparticles can be separated from the bulk solution upon exposure to magnetic fields. This is shown in Figure 1C, where CA-SPION nanoparticles separate from solution and migrate towards the glass surface in contact with an external bar magnet. This data shows that these nanoparticles can be controlled using external magnetic force. |
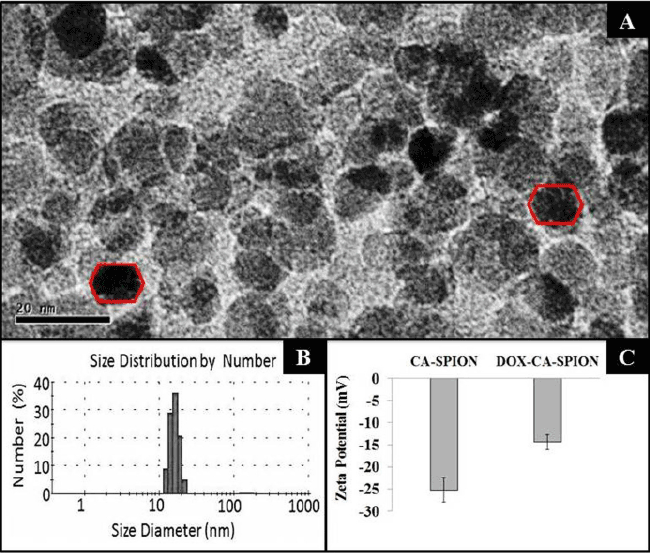
| The TEM images of the synthesized CA-SPION are shown in Figure 2A, showing them to be irregular in size, with an average diameter of about 12 nm. Their size has been further determined by dynamic light scattering (DLS), which measures the hydrodynamic diameter of the nanoparticles. Figure 2B shows the mean hydrodynamic diameter of CA-SPION to be 25 nm. The DLS data are in good accordance with the TEM results, as the DLS determines the hydrodynamic diameter (nanoparticle diameter+hydration layer) of nanoparticles, which is always higher than the actual nanoparticle diameter given by TEM analysis. The surface charge (zeta potential) of CA-SPION, with and without Dox conjugation, is shown in Figure 2C. The zeta potential values of CA-SPION and Dox-CA-SPION are -26.6 mV and -14.6 mV, respectively. This can be explained by the fact that the negative surface charge of CA-SPION, owing to anionic carboxylate groups on their surface, are partially reduced in Dox-CA-SPION by the cationic Dox molecules electrostatically attached to their surface. |
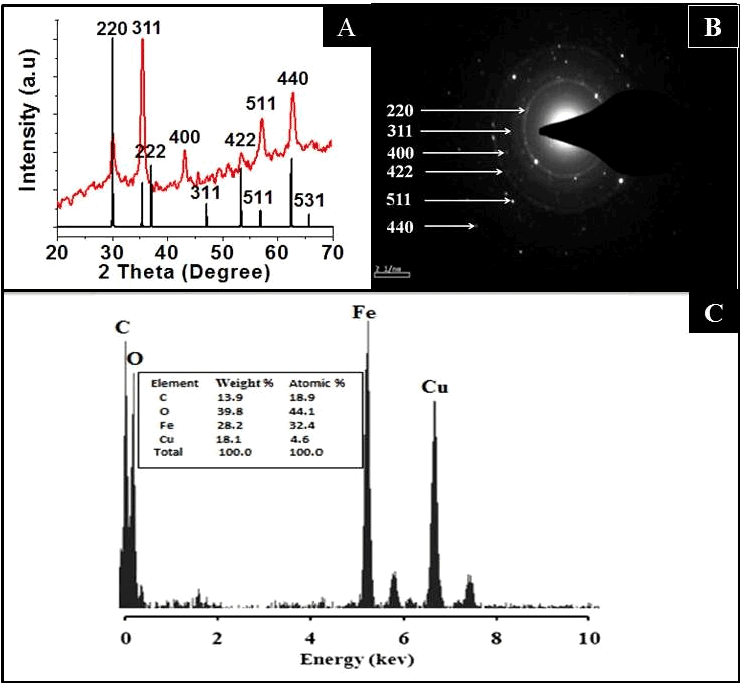
| Figure 3A represents the XRD spectrum of the CA-SPION, showing diffraction peaks of (200), (311), (400), (422), (440) and (500), which match the characteristic peaks of the inverse spinel structure of Fe3O4 (Magnetite, JCPDS card no. 77-1545). The diffraction peaks delineate face-centered lattice (fcc) and cubic unit cells of nanoparticles. The clarity of XRD peaks indicated the crystalline nature of the nanostructures. Figure 3B confirms the crystalline pattern, as observed from the clearly visible diffraction rings in the SAED data. The elemental composition of these nanoparticles is provided in their EDX spectra (Figure 3C), showing the presence of iron and oxygen. The presence of copper and carbon is due to the composition of the grid |
| The surface modification of SPION via coating with CA is shown by the FTIR analysis of SPION, CA and CA-SPION (Figure S1, Supplementary Data). The spectrum of SPION shows the presence of a characteristic peak for Fe-O-Fe as a strong band at 590–605 cm−1. A prominent and broad peak at 3450-3515 cm−1 clearly revealed the presence of numerous surface hydroxyl groups on the surface of SPIONs. The FTIR spectrum of CA shows absorbance peaks at 1668 cm−1 (C=O), 2918 cm−1 (CH2 stretching) and 2918 cm−1 (C–H symmetric stretch), along with one broad peak around 3450 cm−1 (OH-stretching vibration). In the spectrum of CA-SPION, all the peaks match those found in SPION and CA, with the exception of carbonyl (C=O) peak of CA, which has slightly shifted as a result of CA attachment to SPION surface [28,29]. |
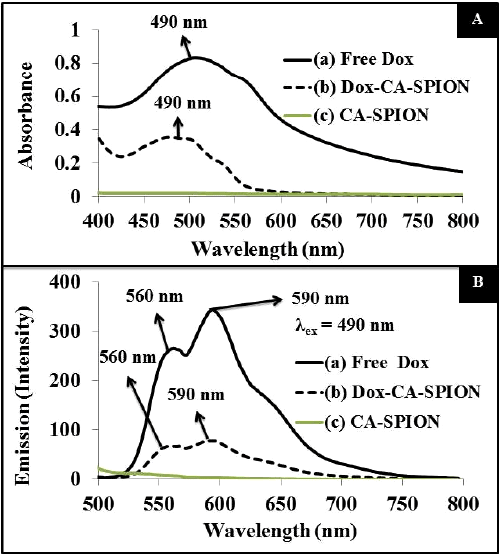
| The optical properties of the Dox-loaded nanoparticles (Dox-CASPION) have been studied by UV-visible and fluorescence spectroscopies. For comparison, the spectrum of free Dox and CA-SPION are also recorded. Figure 4A presents the absorption spectrum, showing that the absorption peak of free Dox (at 490 nm) is reduced and slightly blue shifted upon conjugation with CA-SPION. The same pattern is reflected in the fluorescence spectrum of the above samples, following excitation at 490 nm (Figure 4B). The typical fluorescence spectra of Dox are shown, with peaks at 560 and 590 nm, with most intense peak at 590 nm. The fluorescence intensity of free Dox is significantly reduced upon conjugation with CA-SPION. This can be attributed to the fact that ironoxide is known to quench the fluorescence of fluorophores. Nevertheless, the Dox-CA-SPION sample still has enough optical signal that can be used in bioimaging applications. |
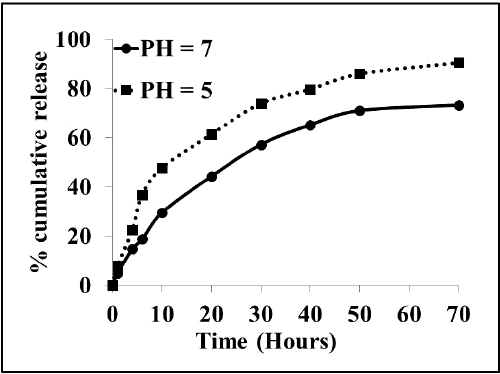
| We next investigated whether the fluorescent drug Dox can be released from the Dox-CA-SPION nanobioconjugates. Figure 5 presents the release kinetic data of the fluorescent drug Dox from the Dox-CA-SPION, at pH 5 and 7 (both at 37ºC). It can be seen that the drug is released from the nanoparticles in a sustained manner, with about 70% and 90% release in 70 hours. This data demonstrates that these nanoparticles can be used as a slow release drug delivery vehicle. |
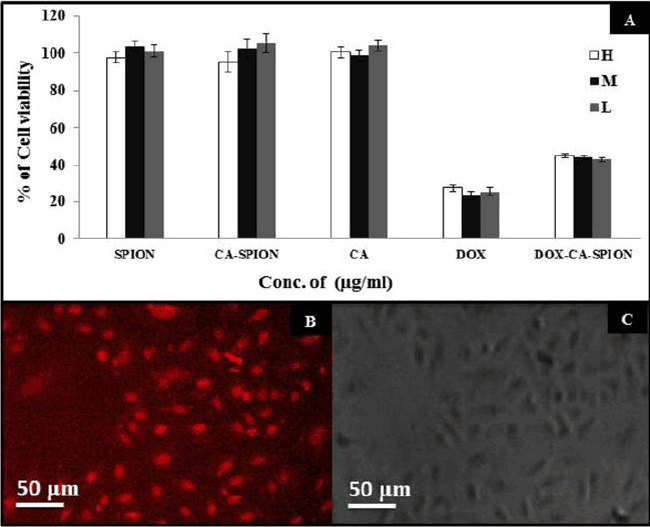
| After synthesis and characterization, we investigated the interaction of these nanoparticles with cells in vitro. First, we ascertained the potential non-specific cytotoxicity of CA-SPION, along with drug induced toxicity of Dox-CA-SPION. For that, we have treated living cells with these nanoparticles and analyzed the cell viability. It can be seen from Figure 6A that after 48 hours of treatment with three different dosages (H: High dose; M: Medium dose; and L: Low dose) of SPION, CA and SPION-CA, the cells remained more than 95% viable, even at a dosage as high as 400 μg/mL. This data demonstrates that the SPION, whether in free form or citric acid coated, exert negligible toxic effects on the cells. However, upon treatment with drug-conjugated nanoparticles (Dox-CA-SPION), significant cytotoxicity has been observed (about 45% cell viability), although it is lower than the cytotoxicity of free Dox (about 25% cell viability). This means that the drug conjugated nanoparticles can function in drug delivery. It is worth noting that free Dox is cardiotoxic, and therefore it can be applied in in vitro experiments only [23]. |
| We also probed the cellular uptake of the Dox-conjugated nanoparticles using fluorescence microscopy of cells, after treatment of 2 hours. The intrinsic fluorescence of Dox was optically tracked. Figure 6B represents the fluorescence images of the treated cells, showing robust optical signal (from Dox) from the cells (pseudo-colored in grayscale). Figure 6C shows the same cells in phase contrast. The treated cells looked morphologically healthy, further indicating the non-toxicity of particles. This experiment shows that the nanoparticles are efficient uptake by the cells, thus highlighting the promise of these nanoparticles also as non-toxic optical labels in bioimaging studies. The above experiments showed that the bioconjugated iron-oxide nanoparticles are passively taken up by cells in vitro, with no sign of non-specific toxicity. |
| Conclusion |
| In this study, we have shown the facile synthesis of drug conjugated superparamagnetic iron oxide nanoparticles in which citric acid could be readily coated to the surface of dox-SPIONs to release the drug in a sustained manner. In vitro studies probed that these nanoparticles have been efficiently up taken by the cells and cell uptake has been further enhanced upon magnetic guidance. CA-SPION nanoparticles were found to be non-toxic while their drug conjugates due to drug induced cytotoxicity were showing less cytotoxicity because of the slow release of the drug. The major therapeutic advantage of drug conjugated nanoparticles in magnetically targeted drug delivery is expected to be more pronounced in in vivo studies where the efficacy of free drug is negligible. Thus, future studies will be focused on optimizing the delivery of CA-SPIONs NPs in vivo using tumor-model animals. |
| Acknowledgements |
| We are grateful to the University of Delhi, India, for providing Research and Development grant to support this study. We thank Ms. Anuradha and Ms. Komal Sethi for their help related to cell culture work. |
References
|
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 12641
- [From(publication date):
March-2016 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 11485
- PDF downloads : 1156
