Research Article Open Access
The Dietary Supplement EGCG: NOPE(N-Oleyl- Phosphatidyletathanolamine and pigallocatechin-3-Gallato Formula)Helps Patients to Follow a Flexible Dietary Regimen and Induces Loss of Weight in Patients who had Previously Experienced No Response to Other Weight Loss Intervention
| Daniele Barbaro*, Anna Menasci, Barbara Baldini, Cristina Pasquini and Paola Lapi | |
| Section of Endocrinology, ASL6 Livorno, Italy | |
| Corresponding Author : | Daniele Barbaro Section of Endocrinology, ASL 6 Livorno Viale Alfieri 36 57100 Livorno, Italy Tel: +390586223017 Fax:+390586223380 E-mail: danielebarbaro@katamail.com |
| Received November 24, 2011; Accepted December 14, 2011; Published December 20, 2011 | |
| Citation: Barbaro D, Menasci A, Baldini B, Pasquini C, Lapi P (2011) The Dietary Supplement EGCG: NOPE ( N-Oleyl-Phosphatidyletathanolamine and Epigallocatechin-3-Gallato Formula) Helps Patients to Follow a Flexible Dietary Regimen and Induces Loss of Weight in Patients who had Previously Experienced No Response to Other Weight Loss Intervention. J Obes Weig los Ther 1:105. doi:10.4172/2165-7904.1000105 | |
| Copyright: © 2011 Barbaro D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background and aims:Obesity is increasing in all countries. The aim of this study was that of evaluating if the treatment with the dietary supplement ( N-oleyl-phstatidylethanolamine and epigallocatechin-3-gallato formula, EGCG-NOPE) could help patients to follow a flexible dietary regimen.
Methods:38 patients (20 males, 18 females, BMI 32-41, aged 33-72 years) were enrolled consecutively. These patients were subjects who had experienced no loss of weight during an intervention program consisting of a short training period, aimed at behaviour modification, followed by a dietary program based on rules of diet, without a fixed amount of calories. These patients were instructed to take EGCG-NOPE, two capsules (EGCG 120 mg and NOPE170 mg each capsule) at 1 hour before lunch and dinner, for two months. The study had a crossover design, and after two months EGCG-NOPE was stopped, the patients continuing to follow only the prescribed dietary rules. After a further two months, patients again took EGCG-NOPE for two more months. The primary end-point of the study was weight loss and a decrease in hip circumference, while the secondary end-point involved metabolic parameters: serum glucose, Homeostasis Model Assessment (HOMA) index, serum LDL and HDL cholesterol and triglycerides.
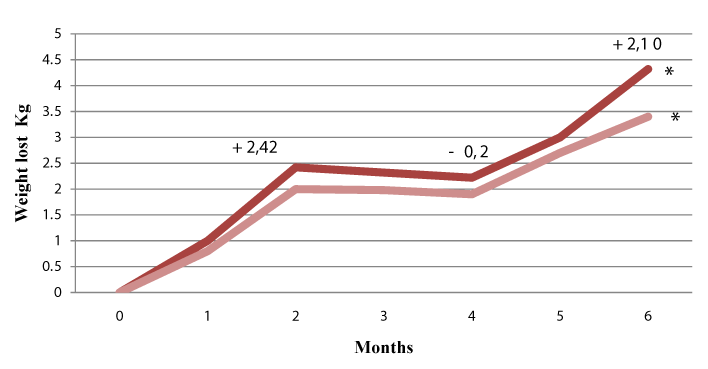
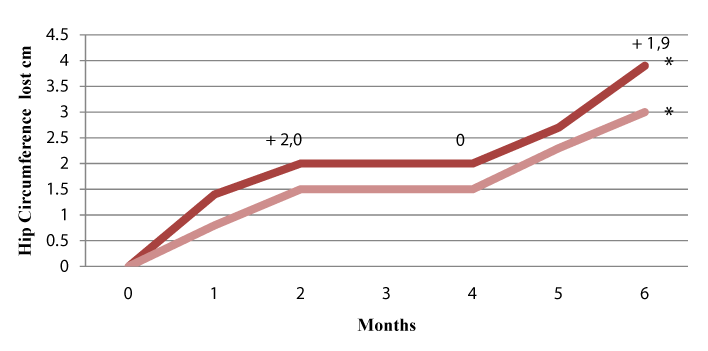
Results: After two months of treatment with EGCG-NOPE, the weight loss was 2.42kg+/-0.3 (p<0.001), and the decrease in hip circumferences was 2.0 cm +/- 0.2 (p<0.001).There was also a decrease in serum glucose (0.083 mm/L p<0.001), HOMA index (0.48 p<0.001), serum LDL cholesterol (0.46 mm/L p< 0.01), and serum triglycerides (0.25 mm/L p<0.001). HDL cholesterol increased by 0.20 mm/L (p<0.01). Following the two months without treatment, the weight and hip circumference were steady. After the next two months of treatment, the patients had a further loss of weight (2.10 kg +/-0.4 p < 0.01) and a further decrease in hip circumference (2.0 +/- 0.1 p < 0.001).
Conclusions: EGCG-NOPE, at these doses, has shown that it is capable of decreasing the body weight and hip circumferences in patients who had failed to lose weight during a previous dietary regimen. The improvement in the metabolic parameters during treatment with EGCG-NOPE represents a further aspect of paramount importa
| Keywords |
| EGCG-NOPE; HOMA index; Dietary supplement |
| Introduction |
| Obesity is increasing in all countries, and represents one of the most concerning problems of public health. Lifestyle modification is the cornerstone of the treatment of obesity, but the short- and long-term effects of conventional weight-loss programs are often unsatisfactory. For this reason, there is an increasing demand for pharmaceutical treatment of this condition [1-4]. Decreasing nutrient absorption, inhibition of appetite and increasing thermogenesis are possible pharmacological methods of treatment. However, most of these have important drawbacks: decreasing nutrients may cause gastrointestinal discomfort, while inhibition of appetite usually involves action affecting the brain structure, with important side effects [5]. To date, no safe drug inhibiting the appetite or increasing thermogenesis is available. For these reasons, there is now great interest in dietary supplements and there are some important papers showing that green tea cathechins can have beneficial effects on weight loss and can improve some metabolic abnormalities in obese subjects. |
| Recently, a dietary supplement made up of two natural active substances, epigallocathechin3-gallate (EGCG) and N-oleylphosphatidylethanolamine (NOPE), has been developed and marketed, and EGCG -NOPE has been shown to be capable of improving compliance with a low-energy diet [6]. A recent randomized double-blind study has shown that the intake of EGCG-NOPE complex improved compliance with diet in a group of healthy overweight or obese patients. All patients treated with EGCG-NOPE experienced an increased feeling of fullness, less severe binge eating, and fewer depressive symptoms during a weight-reduction program based on a restriction of daily energy intake of 3344kJ/d calories less than the daily requirements according to WHO criteria [6]. However, the problem of long-term compliance is not only related to a feeling of well-being in patients, but also to the difficulty of maintaining a diet with a fixed number of calories, as well as many patients being bothered by having to weigh foods and adhere to fixed recipes. |
| For this reason, the aim of this study was not that of evaluating patients who were obliged to follow a predetermined low-calorie diet, but patients freely eating what they wanted, and only following dietary rules after a short training period regarding types of foods and lifestyle. |
| Patients, Methods, and Design of the Study |
| The study was approved by the Ethics Committee of our hospital and thirty-eight patients were consecutively enrolled: 20 males (aged 39-67 years, BMI 33-41) and 18 females ( aged 39-72 years, BMI 32- 40). Enrolled patients were subjects who had experienced no loss of weight during multiple previous treatments by "herbal" and/or other dietary supplement associated to dietary weight reduction program based on a restriction of daily calories. Moreover all these patients had experienced no loss of weight (or a loss of weight <0.5 kg/month) during our program intervention consisting of a short training period aimed at behaviour modification, and a further short training period regarding types of food, followed by a dietary program based on rules of diet without a fixed amount of calories (Table 1). General rules concerning lifestyle were also imparted. Patients were followed weekly for the first month, and then monthly, to evaluate adherence to the dietary program. The 38 patients who had failed to show weight loss, as described above, represented 31% of all patients seen over a one month period. Regarding these patients, an analysis of their dietary diary revealed that 32 of the 38 exhibited a clear non-adherence to the regimen prescribed. For the remaining 6 patients, there was, apparently, no clear explanation regarding their failure to lose weight, since they reported to have followed the dietary regimen. All subjects, after giving their written consent, were invited to take EGCC-NOPE (EGCG 120 mg and NOPE 170 mg each capsule), 2 capsules at 1 hour before lunch and dinner, while continuing with the same intervention program. The study had a cross-over design, and after two months EGCG-NOPE was stopped, with patients only continuing to follow the prescribed dietary rules. After a further two months, patients again took EGCGNOPE as before. The primary end-point of the study was weight loss and decrease oh hip circumference, while the secondary target involved metabolic parameters: blood glucose, Homeostasis Model Assessment (HOMA) index, serum LDL and HDL cholesterol, and triglycerides. Weight loss was assessed at the beginning of the study and then monthly, while the above-stated metabolic parameters were assessed at the beginning of the study and then after two, four, and six months (Figure 1). HOMA index was calculated, following the literature, as fasting plasma insulin (microU/ml) x fasting blood glucose (mmol/L)/22.5. Variation of anthropometric variables and variation of metabolic parameters are reported as the differences registered in respect to the previous values. |
| The recommended rules of diet are reported in Table 1. |
| Criteria of exclusion from the study were mental disease, diabetes mellitus treated with insulin, or severe kidney or liver failure, hypothyroidism of new diagnosis or, if under treatment with L-thyroxine, with TSH increased. |
| Statistical Analysis |
| Data were calculated as differences registered in respect to previous values, and reported as mean (+/- ES), and statistical analysis were performed by Anova test. |
| Results |
| After two months of treatment with EGCC-NOPE, the weight loss was 2.42 kg+/- 0.3 (p<0.001), and the decrease in hip circumference was 2.0 cm +/- 0.2 (p<0.001). There was a decrease in serum glucose of 0.83+/-0.06 mm/L (p<0.001), a decrease in the HOMA index of 0.48+/-0.03 (p<0.001), serum LDL cholesterol decreased by 0.45+/- 0.04 mm/L (p<0.01), serum HDL cholesterol increased by 0.20+/-0.01 mm/L ( p<0.01), and triglycerides was reduced by 1.14+/-0.20 mm/L( p<0.001). No side effects were reported, and patients experienced a greater ability to follow the dietary regimen. Following the two months without treatment, the weight and metabolic parameters were steady, apart from the HOMA index, which increased by 0.20+/-0.01(p<0.01). After the next two months of treatment, the patients had a further weight of loss 2.10 +/-0.2 (p<0.001), with a further reduction in the HOMA index of 0.42 +/-0.03 (p<0.001), and a further reduction in triglycerides of 0.25+/-0.03 mm/L (p<0.001). No statistical variation was shown for serum glucose or LDL and HDL cholesterol. Clinical data at the various stages are plotted in Figures 1 and 2. Also plotted in Figures 1 and 2 are clinical data of the subgroup of patients who reported to have carefully kept to the dietary regimen and lifestyle modifications. The metabolic parameters are summarized in Table 2. No patients experienced adverse side effects. |
| Discussion |
| In any long-term diet, compliance of the patients represents the crucial point for the successful treatment of obesity. A failure in compliance by patients with the dietary regimen may be related to a reduced feeling of well-being, to severe binge eating, or to mood depression. Moreover, a fixed hypocaloric diet can produce a paradoxical effect on the appetite, resulting in compulsive hunger. All these aspects are often worsened by the objective difficulty of cooking fixed recipes with a fixed amount of calories [1-4,7,8]. |
| For these reasons, our approach, in patients supposed to be capable of changing their dietary behaviour, is usually to impart training regarding types of foods, followed by dietary rules without any fixed daily caloric intake. Among a group of patients who were subjects in this program, but who were unable to lose weight, we investigated the effect of EGCG-NOPE. Since the literature reports the use of green tea catechins till to 1000 mg daily [20-24] we decided to use ECGC-NOPE in a greater with respect to the previous paper [6]. |
| In our study, ECGC-NOPE was shown to be capable of inducing patients who had previously experienced failure to adhere to this flexible dietary regimen. |
| The possible mechanism for these results can be found, according to the literature, in the effects of the special formulation of this compound. |
| In fact, both EGCG and NOPE, which is a precursor to the natural compound NOE, administered separately via an oral supplement, have a low level of bioavailability. Vice versa, the compound form EGCG-NOPE has been shown to ameliorate bioavailability in rats [6], by a dual mechanism; in fact, EGCG has a higher degree of intestinal permeability, and NOPE is protected against digestive- induced hydrolysis. The effect of NOPE-derived NOE on weight loss can have a number of explanations [6]. |
| NOE is produced primarily in the small intestine, acting mainly through the receptor PPAR alpha, and it has been proposed that the resulting reduction in nitric oxide, PPAR alpha-mediated, could lead to stimulation of vagal afferents and, consequently, to reduction in food intake [9-11]. Moreover, NOE also acts as an agonist in gastrointestinal orphan receptor GFR 119, that is involved in satiety regulation [12]. |
| Along with these NOE-induced effects primarily acting on satiety, an EGCC action on body-fat mass has been postulated. |
| Data on animals suggest that EGCG can increase fat oxidation both at rest and during physical activity, and that it can reduce body fat [13,14]. These results have not been confirmed in studies on humans [15-19], but in all these quoted studies EGCG was administered alone (not in the formulation EGCG-NOPE), and in a lower amount [16,18,19]. It is impossible to say if our results are merely due to the effects of EGCG-NOPE on satiety, or are also due to a direct effect of prevention of body fat accumulation, or to some lipolytic action. The fact that a small subgroup of patients, in which there was evidence of adherence to a dietary and lifestyle regimen, showed a loss of fat only after treatment with EGCG-NOPE could theoritically support the hypothesis of a direct effect on fat accumulation by this compound, at least as regards the doses used in this study. |
| In a previous study, the beneficial effect of EGCG-NOPE on body weight had already been demonstrated. In that double-blind randomized study, ECGC-NOPE was shown capable of inducing satiety, of decreasing binge eating, and of ameliorating mood compared against a placebo. Treated patients demonstrated an improved sense of well-being during a fixed-diet, energy-restriction regimen of 3344 kJ/d, with the conclusion of the study being that EGCG-NOPE could increase compliance with the diet. In this study, a half dose of EGCG-NOPE was used, and the loss of weight recorded was not statistically different between the two groups. |
| Our study allowed us to demonstrate that EGCG-NOPE can be effective even without prescribing a fixed hypocaloric diet, and this can be a great advantage for a long-term dietary regimen. We investigated neither binge eating severity nor depressive symptoms because all patients had already failed the previous dietary regimen and the fact that EGCG-NOPE induced weight loss is the best evidence indicating the improved well-being of these subjects. As already stated, a further effect could, of course, be a direct action of this compound, at these doses, on fat accumulation. |
| Another important action of EGCG- NOPE could be, as shown in the already-quoted study, the effect of EGCG on insulin sensitivity is as much as it is well documented that catechins improve insulin action. Our data corroborate this, and, in fact, the results suggest that the effects on insulin resistance are not merely due to the loss of weight, but appear to be related to a direct effect of EGCG-NOPE on insulin sensitivity. Our study was not a blind study and for this reason we can't completely rule out a placebo effect of the capsules of EGCG-NOPE. However this hypothesis, in our opinion, appears remote because all these patients were enrolled because they had previously experienced multiple failures during ingestion of herbal or dietary supplement. So, we can suppose that in our group placebo effect was negligible. Moreover the modifications of HOMA index have appeared to be not related strictly to variation of weight, during the period of stoppage of EGCG-NOPE the weight was steady but the HOMA index increased significantly. |
| Our results regarding metabolic parameters have shown not only an improvement in the HOMA index, but also an improvement in lipid profiles. Insulin resistance and the "low grade" inflammatory status, which are usually strongly related, can affect serum lipids, and can especially increase LDL cholesterol and decrease HDL cholesterol. Our study doesn't permit us to say if the effect on lipids is a result of direct action on the lipids or is a secondary effect coming from the decrease in insulin resistance, but, without doubt, the improvement in the metabolic parameters represents primary benefits for obese patients. |
| Conclusions |
| EGCG-NOPE appears to bring about several beneficial effects in obese patients. Using fixed doses of EGCG-NOPE (EGCG 480 mg/day and NOPE 680 mg/day), our study has shown that patients can lose weight when treated employing a flexible dietary regimen, and this may represent a great benefit for those subjects who, for various reasons, exhibit a low degree of compliance with fixed hypocaloric diets. During the two months of stoppage of the treatment, weight remained steady, and this appears to be another important datum; moreover, the improvement seen in metabolic profile during the intake of EGCG-NOPE represents a further aspect of paramount importance for obese patients. Whether treatment with EGCG-NOPE can be continued safely, over long periods, and whether it can induce a progressive loss of weight until optimal weight has been achieved, is something to be established by long-term study. |
References
- Kaplan LM (2010) Pharmacologic therapies for obesity. Gastroenterol Clin North Am 39: 69-79.
- Enwald HP, Huotari ML (2010) Preventing the obesity epidemic by second generation tailored health communication: an interdisciplinary review. J Med Internet Res 12: e24.
- Delahanty LM (2010) An expanded role for dietitians in maximizing retention in nutrition and lifestyle intervention trials: implications for clinical practice. J Hum Nutr Diet 23: 336-343.
- Aphramor L (2010) Validity of claims made in weight management research: a narrative review of dietetic articles. Nutr J 9: 30.
- Rao G (2010) Office-based strategies for the management of obesity. Am Fam Physician 81: 1449-1456.
- Rondanelli M, Opizzi A, Solerte SB, Trotti R, Klersy C, et al. (2009) Administration of a dietary supplement ( N-oleyl- phosphatidylethanolamine and epigallocatechin-3-gallate formula) enhances compliance with diet in healthy overweight subjects: a randomized controlled trial. Br Journal Nutr 101: 457-464.
- Pittler HM, Ernst E (2005) Complementary therapies for reducing body weight: a systematic review. Int J Obes (Lond) 29: 1030-1038.
- Melin I, Reynisdottir S, Berglund L, Zamfir M, Karlstrom B (2006) Conservative treatment of obesity in an academic obesity unit. Long-term outcome and dropout. Eat Weight Disord 11: 22-30.
- Nielsen MJ, Petersen G, Astrup A, Hansen HS (2004) Food intake is inhibited by oral oleoylethanolamide. J Lipid Res 45: 1027-1029.
- Fu J, Gaetani S, Oveisi F , Lo Verme J, Serrano A, et al. (2003) Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425: 90-93.
- Broccali G, Berti M, Pistolese E, Cestaro B (2005) Hydrolyzed milk-serum peptides reduce body weight and fat content of dietary obese rats ameliorating their antioxidant status and liver functions. Panminerva Med 47: 123-129.
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, et al. (2006) Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 3: 167-175.
- Chen L, Lee Mj, Li H, Yang CS (1997) Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos 25: 1045-1050.
- Kao YH, Hiipakka RA, Liao S (2000) Modulation of endocrine system and food intake by green tea epigallocatechin gallate. Endocrinology 141: 980-987.
- Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, et al. (1999) Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 70: 1040-1045.
- Williamson G, Manach C (2005) Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 81: 243S-255S.
- Higdon JV, Frei B (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 43: 89-143.
- Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, et al. (2007) Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr 26: 396S-402S.
- Brown AL, Lane J, Coverly J, Stocks J, Jackson S, et al. (2009) Effects of dietary supplementation with the green tea polyphenol epigallocatechin - 3 - gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr 101: 886-894.
- Sae-Tan S, Grove KA, Lambert JD (2011) Weight control and prevention of metabolic syndrome by green tea Pharmacol Res. 64: 146-154.
- Rains TM, Agarwarl S, Maki KC (2011) Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem 22: 1-7.
- Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, et al. (2010) Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome J Am Coll Nutr 29: 31-40.
- Westerterp-Plantenga MS (2010) Green tea catechins, caffeine and body weight regulation. Physiol Behav 100: 42-46.
- Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, et al. (1999) Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24 - h energy expenditure and fat oxidation in humans. Am J Clin Nutr 70: 1040-1045.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 6439
- [From(publication date):
December-2011 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 1855
- PDF downloads : 4584
