Research Article Open Access
Variety of Neuronal Pathways to Achieve the Same Hypoxic Preconditioning Effect
Elena I Zakharova* and Alexander M Dudcheko
Laboratory of General Pathology of Cardiorespiratory Systems, Institute of General Pathology and Pathophysiology, Moscow, Russia
- Corresponding Author:
- Elena I Zakharova
Laboratory of General Pathology of Cardiorespiratory Systems
Institute of General Pathology and Pathophysiology, Moscow, Russia
Tel: 0079199668657
E-mail: zakharova-ei@yandex.ru
Received date: November 28, 2016; Accepted date: November 29, 2016; Published date: December 20, 2016
Citation: Zakharova EI, Dudcheko AM (2016) Variety of Neuronal Pathways to Achieve the Same Hypoxic Preconditioning Effect. Biochem Physiol 5:211. doi:10.4172/2168-9652.1000212
Copyright: © 2016, Zakharova EI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
The relevance of hypoxic preconditioning is due to its ability to increase the body's resistance to hypoxic/ischemic stress. A single session of moderate hypoxia eliminates the differences in endurance under severe hypoxia in intact rats and those pre-tested under severe hypoxia with high and low innate resistance to it. In these rat groups, the same preconditioning effect is achieved by different synaptic plastic tools (biochemical data). Thus, the synaptic mechanisms of preconditioning are dependent on the prior hypoxic experience. This conclusion confirmed our pharmacological experiments. Antagonists of alpha7 and non-alpha7 subtypes of the nicotinic receptors methyllycaconitine and mecamylamine in single IP injections selectively influenced the resistance to hypoxia of the low-resistant rats and were ineffective against the high-resistant and intact rats. Moreover, in the low-resistant rats, both drugs had ambiguous effects on the resistance to hypoxia after the preconditioning and without it. Based on the data, we substantiated in our review that the following cholinergic neuronal populations and networks were involved in mechanisms of the hypoxic preconditioning: 1) In the high-resistant rats, cholinergic projections from the pedunculopontine and/or laterodorsal tegmental nuclei into the nuclei of the ventrolateral medulla of the medulla oblongata as well as into the subcortical forebrain nuclei and, linked with them, cholinergic projections from these forebrain nuclei into the cortex. 2) In the low-resistant rats, cholinergic C-fibres into the nucleus tractus solitary of the medulla oblongata and influences of unidentified cholinergic neurons through the alpha7 nicotinic receptors in the caudal brainstem areas outside the nucleus tractus solitary. 3) In intact rats, cortical cholinergic interneurons and unidentified cholinergic neurons of the brainstem structures. In conclusion, the variety of neuronal pathways to put off apnea indicates a great adaptive potential of brain, and the specific mechanisms of its realization may be a promising therapeutic targets.
Keywords
Severe hypoxia; Apnea; Hypoxic preconditioning; Caudal brainstem; Cortex; Neuronal networks; Cholinergic system; Alpha7 and non-alpha7 nicotinic receptors; Synaptic choline acetyltransferase
Abbreviations
Ach: Acetylcholine; C1: Area of premotor sympathetic neurons; ChAT: Choline Acetyltransferase; DFA: Dorsal Facial Area; HBH: Moderate Hypobaric Hypoxia; IC: Intracistermal; IP: Intraperitoneal; LDT: Laterodorsal Tegmental Nucleus; mAChRs: Muscarinic Cholinergic Receptors; MCVA: Medullary Cerebral Vasodilator Area; MEC: Mecamylamine; MLA: Methyllycaconitine; nAChRs: Nicotinic Cholinergic Receptors; NTS: Nucleus Tractus Solitary; PPT: Pedunculopontine Tegmental Nucleus; SHBH: Severe Hypobaric Hypoxia; T: Survival time under SHBH conditions; VLM: Ventrolateral Medulla.
Introduction
The relevance of hypoxic preconditioning is due to its ability to increase the body's resistance to hypoxic/ischemic stress. Hypoxic factor is the main in ischemic preconditioning effect. It is likely that hypoxic component forms the pathogenesis of many diseases, and a study of the preconditioning mechanisms is a high priority [1-4].
In the problem of hypoxic adaptation, the brain is central, not only as the most sensitive organ to hypoxia, but also as the coordinator of functions of all body organs and systems. In the nervous tissue, the functional specificity and individual sensitivity to hypoxia of separate neuronal populations and the corresponding brain structures is of fundamental importance. The structures of forebrain are most unstable to ischaemic/hypoxic injuries [5]. The most interesting are the cortex and hippocampus, as these are the higher brain structures responsible for cognitive functions and complex behaviour.
Under moderate hypoxia conditions (10-12% O2 for rat) which are parts if hypoxic training, the key role in the adaptive transformations of the body functions belongs to the autonomic respiratory and cardiovascular systems. Their central re presentation is located in the medulla oblongata and pons varolii (caudal brainstem). Autonomic systems are functionally closely interrelated by the “respiratory centre”, groups of respiratory neurons which support respiratory rhythm [6-8]. Both the cortex and hippocampus interact with the cardiorespiratory systems, participating in the regulation of voluntary breathing and supposedly adaptive reactions of the cardiovascular and respiratory systems [7-10].
The study of neuronal networks of the central autonomic regulation of breathing and blood circulation, and the mediator and functional specificity of their components in health and disease is the focus of many researchers because of the basic value of this knowledge to maintain the vitality of the body. Starting from Loeschcke studies [11,12], the central effects of acetylcholine (ACh) and its analogues on the respiration and blood circulation are intensively investigated. Cholinergic participation is detected in the majority of the functional sites of cardiorespiratory networks, as well as the ambiguity of the cholinergic effects depending on drug application site, reception and dose [13-23].
It was later shown in various organs including the brain that ACh simulates the effects of ischaemic/hypoxic preconditioning which are usually realised through nicotinic receptors (nAChRs) [24-27]. Homomeric alpha7 and heteromeric alpha4beta2, alpha3beta4 and alpha3beta2 are the most widespread nAChR subtypes in the mammalian brain [28]. NAChRs are expressed at all levels of autonomic regulation of respiration and blood circulation from peripheral organs [25,27,29-31] to the central sensory, respiratory and motor neurons [17,20,22,30,32-34].
In our investigations, single moderate hypobaric hypoxia (HBH) significantly increases a resistance to severe hypobaric hypoxia (SHBH) in intact rats and high- and low-resistant, pre-tested to SHBH rats [35,36]. Our data confirm the obligatory participation of the brain stem autonomic systems in the HBH precondition mechanisms. The synaptic pool of the caudal brainstem reacts to HBH in all groups of rats unlike those of the cortex and hippocampus (biochemical data) [37]. At the same time, on the one hand, we found that HBH equalizes the resistance to SHBH in all rat groups [36] and on the other hand, that the same preconditioning effect is achieved by different synaptic plastic tools [37].
Moreover, this response to HBH is observed in different populations of pre-synapses [37]. It assumes the involvement of different populations of cholinergic neurons and hence of different neuronal networks in the preconditioning mechanisms in rats with different innate resistance to severe hypoxia and prior hypoxic experiences. The assumption is evidenced by our early pharmacological data in the high- and low-resistant rats. We got the differential effects on HBH of antagonists of alpha7 and non-alpha7 subtypes of nAChRs methyllycaconitine (MLA) and mecamylamine (MEC), respectively, in these rat groups [35]. However, some of the pharmacological data needs to be clarified.
Therefore, the purpose of our manuscript is to review and reasoning of cholinergic neuronal pathways of the HBH preconditioning realization in the intact, high-resistant and low-resistant rats. For the review process, our additional experimental data on the effect of MLA and MEC on resistance to SHBH and on preconditioning efficiency of HBH on resistance to SHBH, and also preceding biochemical data on the reaction on HBH of the activity of choline acetyltransferase (ChAT) in synaptic pool of the caudal brainstem and cortex in all three rat groups are presented in the manuscript.
Materials and Methods
Animals and ethical policy
Experiments were performed on male outbred albino rats aged 2-2.5 months (weight 200-250 g) at the beginning of the study. The rats were supplied from the animal nursery “Light Mountains” (Russian Federation) and then kept in the vivarium of the Institute of General Pathology and Pathophysiology.
All animal care and experimental procedures were conducted in accordance with the official regulations of the European Communities Council Directive on the use of laboratory animals of 24 November, 1986 (86/609/EEC). The study was approved by the Ethical Committee of the Institute of General Pathology and Pathophysiology. All efforts were made to minimize the number of animals used and their suffering.
The rats were housed in a temperature-controlled room (20–24°C) with 5-7 animals per cage, with free access to food and water, and maintained with a 12 h light/dark cycle. The rats were handled for at least two consecutive days prior to being placed in the pressure chamber. At the end of the experiment, the animals were killed by inhalation of CO2 euthanasia using apparatus for euthanasia AE0904 (Open Science, Russian Federation).
Hypoxic models
We used the same hypoxic models as before [35,37,38]. Hypoxia of varying severity was created in the pressure chamber. The barometer of the chamber (altitude gauge) was calibrated to an altitude above sea level. The rats in the chamber "were raised" at a speed of 50 m/s to the adaptive altitude of 5000 m (HBH, equivalent to 11% O2, 60 min) or to the critical altitude of 11500 m (SHBH, equivalent to 4.5% O2). In the latter test, resistance to hypoxia was recorded with respect to time (T) under SHBH conditions that was time until agonal inspiration (apnea) in combination with a loss of voluntary control of body tone.
Drug application
The rats received a single IP injection of methyllycaconitine citrate (MLA, Molecular Weight: 874.93, Tocris), which is a selective antagonist of alpha7 subtype of nAChR, or mecamylamine hydrochloride (MEC, Molecular Weight: 203.75, Sigma), which is a selective antagonist of non-alpha7 subtypes of nAChRs (preferential antagonist of alpha4beta2 and alpha3-containing nAChRs). Respectively, MLA (1.4 nmol/kg) or MEC (3.9 nmol/kg) was injected immediately (for MLA) or 30 min (for MEC) before SHBH or HBH. The time of treatment was evaluated from the pharmacokinetics of these drugs [39-42]. The drugs were dissolved in sterile isotonic (physiological) saline. The control to drugs rat groups received physiological saline.
Experimental protocol
Some of the rats were pre-tested under SHBH conditions and the values of innate T (T1) were estimated. The animals were divided into groups of low and high resistance to hypoxia with T1<3.5 min and T1>7 min respectively. For the following 4-5 weeks, all rats were kept under standard vivarium conditions, after which the high-, lowresistant and not pre-tested intact rats were subdivided into the HBH +SHBH and SHBH drug groups.
The rats of SHBH drug groups received MLA or MEC injections and then were subjected to SHBH. It was the first SHBH exposure for intact rats and second for pre-tested high- and low-resistant rats and values of T1 and T2 were estimated, respectively.
The rats of HBH+SHBH drug groups received MLA or MEC injections and then were subjected to a single HBH session; four min after the end of the session, the rats were subjected to SHBH. In the same manner as for the SHBH drug groups, values of T1 for the intact rats and T2 for the high- and low-resistant rats were estimated.
Each drug group had a control group. The control groups duplicated the drug groups, but control rats received saline instead drugs. All of the experiments with the drugs were tested simultaneously to the corresponding control group.
All data were generated in a double blind manner which has been achieved thanks to the technical assistance of our colleague.
Statistics
The data were calculated using the non-parametric one-sided Fisher’s Exact Test and the r-criterion of the Pearson’s correlative test in Microsoft Excel with the formula being adjusted for a small number of observations (n=4-15) [43]. Differences were considered to be statistically significant if p<0.05. According to the Bonferroni correction, differences were considered to be statistically significant if p<0.025 for four selections and if p<0.02 for more than four selections. In last cases, the significance level p<0.05 was considered a tendency to the validity of events and was taken into account in the presence of other ways of comparisons.
For the statistical analysis were also used previously published biochemical data on choline acetyltransferase (ChAT, EC 2.3.1.6) activity in synaptic membrane and synaptoplasm sub-fractions (membrane-bound and water-soluble ChAT, respectively) of “light” and “heavy” synaptosomes isolated from the caudal brainstem (medulla oblongata and pons varolii) and cortex of the intact, highresistant and low-resistant HBH rat groups. Synaptic tools of cholinergic reaction in these brain structures on HBH were analysed in detail [37]. However, the correlation between the response of the synaptic pool in the caudal brainstem and cortex were not analysed.
Results and Discussion
Action of MLA and MEC on direct and HBH-mediated resistance to SHBH in the intact, high and low resistant rats
Pharmacological data
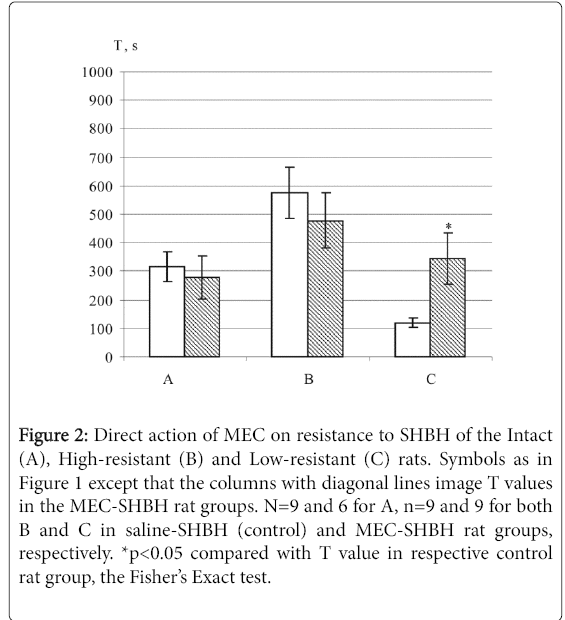
In the intact and high-resistant SHBH drug rat groups, both MLA and MEC had no effect on the direct SHBH action (Figures 1 and 2). In the low-resistant rats, both MLA and MEC potentiated the direct resistance to hypoxia and T2 values in the SHBH drug groups significantly different from the T2 values in the SHBH control groups (Figures 1C and 2C).
Figure 1: Direct action of –?–?–ź on resistance to SHBH of the Intact (A), High-resistant (B) and Low-resistant (C) rats. T (s), endurance under SHBH exposure. Values are expressed as means ± SE. In each pair of columns: left, white columns, T values in the control saline- SHBH rat groups (T1 for A, n=9, and T2 for B and C, n=7 and 10, respectively); right columns with diagonal lines, T values in the MLA-SHBH rat groups (as in the respective control groups, T1for A, n=6, and T2 for B and C, n=6 and 5, respectively). **p<0.025 compared with T value in respective control rat group, the Fisher’s exact test.
Figure 2: Direct action of –?EC on resistance to SHBH of the Intact (A), High-resistant (B) and Low-resistant (C) rats. Symbols as in Figure 1 except that the columns with diagonal lines image T values in the MEC SHBH rat groups. N=9 and 6 for A, n=9 and 9 for both B and C in saline-SHBH (control) and MEC-SHBH rat groups, respectively. *p<0.05 compared with T value in respective control rat group, the Fisher’s Exact test.
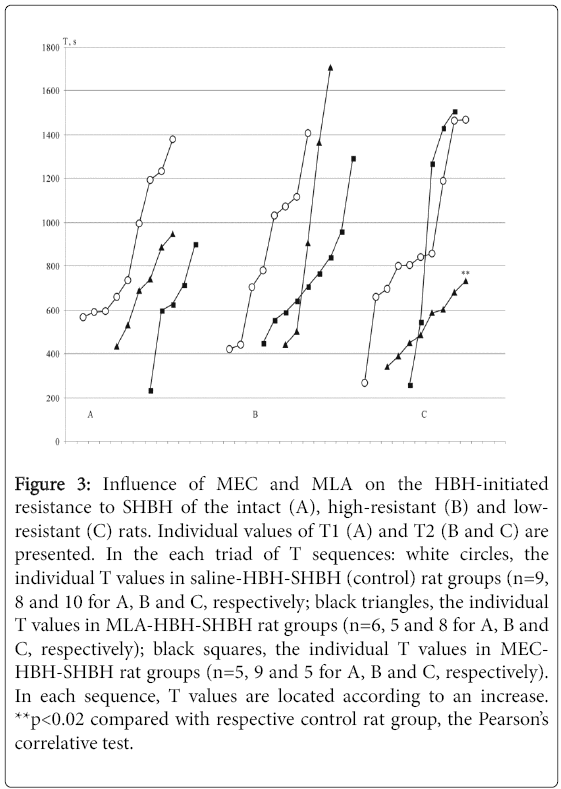
HBH removes the direct effects of SHBH. After HBH sessions, all HBH-SHBH control rat groups show a similar range of values for resistance to SHBH with mean T values of 14.7 ± 1.7, 14.9 ± 1.7 and 13.2 ± 1.8 min vs. mean T values under direct SHBH exposure 5.2 ± 0.9, 10.3 ± 2.2 and 2.6 ± 0.5 min in the intact, high- and low-resistant rat groups, respectively. Thus, HBH equalizes the resistance to SHBH between all rat groups, forming them the same variational series [36] (Figure 3, the sequences of white circlets in A, B and C).
Figure 3: Influence of MEC and MLA on the HBH-initiated resistance to SHBH of the intact (A), high-resistant (B) and lowresistant (C) rats. Individual values of T1 (A) and T2 (B and C) are presented. In the each triad of T sequences: white circles, the individual T values in saline-HBH-SHBH (control) rat groups (n=9, 8 and 10 for A, B and C, respectively; black triangles, the individual T values in MLA-HBH-SHBH rat groups (n=6, 5 and 8 for A, B and C, respectively); black squares, the individual T values in MECHBH- SHBH rat groups (n=5, 9 and 5 for A, B and C, respectively). In each sequence, T values are located according to an increase. **p<0.02 compared with respective control rat group, the Pearson’s correlative test.
The action of MLA and MEC on HBH-induced resistance to SHBH showed some specifics, and as before in the low-resistant rats (Figure 3C). MLA only but not MEC had an influence on the resistance to SHBH after HBH exposure. MLA twice narrowed the preconditioning effect of HBH, reducing the upper limit of resistance and without affecting its lower border. In the intact and high-resistant HBH-SHBH drug rat groups, both MLA and MEC had no significant effect on HBH-induced resistance to SHBH (Figures 3A and 3B).
These data confirmed previously obtained results in respect to lowresistant HBH-SHBH drug rat group but did not confirm the tendency to the decay of resistance to hypoxia in the HBH-SHBH drug group of high-resistant rats after the administration of MEC [35]. From this data, it can be seen that alpha7 and non-alpha7 nAChRs prevented the formation of resistance to hypoxia at the direct SHBH, conversely, alpha7 nAChRs were needed for the maximal HBH effects and nonalpha7 nAChRs were removed from the preconditioning mechanisms in the low-resistant group of rats. The differences in reactions following the administration of the nAChR antagonists under direct and HBHmediated conditions in the rats within the low-resistant rats pointed to the different nicotinic mechanisms of HBH-initiated resistance to hypoxia in relation to direct resistance.
In the intact rats, as in the high-resistant rats, a lack of response to selective action of MLA on alpha7 and MEC on non-alpha7 nAChRs was in both hypoxic protocols. It should be noted that the intact group is a mixture of comparable numbers of rats with innate low, high and intermediate resistance to hypoxia [36] and therefore, the effects of selective antagonists on rat resistance could not be manifested.
It is important that both antagonists of nAChRs were injected in the low doses of 1.4 (MLA) and 3.9 (MEC) nmol/kg corresponding to Ki of these drugs. Such doses guarantee the selectivity of their action on nAChRs. Moreover, the effectiveness of low doses of both antagonists is reason to assume that their actions on the resistance to hypoxia in both experimental protocols were central. It is known that both MEC and MLA penetrate the blood-brain barrier and their central action occurs in much smaller concentrations compared with their peripheral action [42,44,45]. The central action within the medulla oblongata is proved by comparison of the effectiveness of the systemic (intravenous), intracerebroventricular and intracistermal (IC) administration of MLA and some other selective agonists and antagonists of alpha7 and alpha4beta2 nAChRs on the cardiovascular functions at much higher doses [18].
Therefore, we analysed the possible medullary areas of targets for MEC and MLA in both our experimental protocols of the hypoxic exposures. An additional argument was received in respect to MLA, whose participation in precondition mechanisms has been identified only in the low resistance rats. In this group of rats, the synaptic pool reacted to HBH exposure in the caudal brainstem only.
Reaction on HBH of the ChAT activity and protein content in the subsynaptic fractions of the caudal brainstem and cortex of the intact, high- and low-resistant rats
Biochemical data
In the biochemical experiments, the rats were taken 4 min after the end of the HBH session, i.e., biochemical indicators as much as possible reflect the preconditioning effect on the synaptic pools of caudal brainstem, cortex and hippocampus in the intact, high- and low-resistant rats [37].
In the hippocampus, HBH does not initiate any changes in the synaptic parameters of tested rat groups. In the cortex, the cholinergic reaction on HBH is revealed in the synaptic pools of intact and highresistant rat groups. In the caudal brainstem, the cholinergic synaptic pool of reacts to HBH in all three groups of rats and it is the sole brain region which reacts in the low-resistant rats [37]. This confirms the obligatory participation of the brainstem autonomic systems in the HBH precondition mechanisms. The changes in ChAT activity under HBH in the caudal brainstem and cortex are presented in Table 1.
| Rat group | Caudal brainstem | Cortex | ||
| Light | Heavy | Light | Heavy | |
| synaptosomal | synaptosomal | synaptosomal | Â synaptosomal | |
| fraction | fraction | fraction | fraction | |
| %; sub-fraction | %; sub-fraction | %; sub-fraction | %; sub-fraction | |
| Intact (n=6) | 127±8**; sp | 67±10**; sm | No significant changes | 128±8*; sp 109±6; sm |
| r | -0.962## | +0.954## | ||
| High-resistent (n=3) | 81±8*; sp | No significant changes | 67±5*; sm | No significant changes |
| Low-resistant (n=5) | No significant changes | 83±8**; sp | No significant changes | No significant changes |
Table 1: Reaction to HBH of the cholinergic synaptic pool in the caudal brainstem and cortex of the intact, high-resistant and low-resistant rats1.
Synaptic plastic tools of the hypoxic preconditioning in these brain structures are analysed in detail and justified by the following reliable morphological and functional changes of synaptic parameters [37]. 1) In the intact rats, 1a) In the synaptic pool of caudal brainstem, transformation of the pre-synapses of the heavy fraction of synaptosomes with border density in pre-synapses with lower density and for this reason transfer when fractionated into the light fraction of synaptosomes, and activation in the transformed pre-synapses of synthesis and non-quantum leakage of Ach. 1b) In a similar manner in the synaptic pool of cortex but for pre-synapses of the heavy synaptosomal fraction with larger density, the transformation within the heavy fraction and activation in transformed pre-synapses of the synthesis and as a result non-quantum leakage of Ach. 2) In the highresistant rats, 2a) Inhibition of synthesis and depression of the ACh non-quantum leakage in the caudal brainstem (the light fraction of synaptosomes). 2b) Inhibition of the quantum secretion of ACh in the cortex (the light fraction of synaptosomes). 3) In the low-resistant rats, inhibition of synthesis and depression of the ACh non-quantum leakage in the caudal brainstem (the heavy fraction of synaptosomes).
Purpose of our review was to identify the populations of cholinergic neurons and neuronal pathways for the HBH preconditioning realization. Therefore, we carried out a correlative analysis between the changes in ChAT activity in the caudal brainstem and cortex in the intact and high-resistant rat groups. Relationship between the HBHinitiated changes of the caudal brainstem and cortical ChAT activity was detected in the high-resistant rat group, but not in the intact rat group. In the high resistance rat, valid correlation between caudal brainstem and cortical ChAT activity in the respective synaptosomal sub-fractions was both in the HBH group (r=+1, p<0.05, n=3) and in the total control+HBH group (r=+0.911, p<0.02, n=6). For the intact rats correlation between caudal brainstem and cortical ChAT activity in the respective synaptosomal sub-fractions was nonsignificant in the HBH group (r=+0.417, p>0.05, n=6) and in the total control+HBH group (r=+0.403, p>0.05, n=10).
Formulation of the problem, the same preconditioning hypoxic effect can be achieved by various neuronal pathways
We found that HBH preconditioning eliminates the differences in resistance to SHBH between the intact, high- and low-resistant groups of rats with different innate resistance to severe hypoxia and prior hypoxic experiences [36]. The data outlined in this research indicated different synaptic and receptive cholinergic preconditioning mechanisms in these groups of rats. It showed 1) the opposite changes in ChAT activity in intact rat brain with respect to those pre-testing, 2) ChAT reaction to HBH in different synaptosomal fractions within brain structures in these rat groups, and 3) selective involvement of nAChRs in the preconditioning mechanisms of low-resistant rat group.
This assumed that the same preconditioning hypoxic effect were realised through different populations of cholinergic pre-synapses in different experimental groups of rats and as a consequence can be achieved by various neuronal pathways. Reasoning of these theses is given below.
Sources of cholinergic influences in the cortex and caudal brainstem
Cortex and hippocampus
According to immunocytochemical data, the cortex (and hippocampus) has two main cholinergic sources, namely, a major source is the cholinergic projection neurons from the basal forebrain nuclei, and minor source, the cholinergic interneurons [46-50].
Regarding this and using comparative immunocytochemical and own biochemical data, we previously showed for the cortex and hippocampus that pre-synapses of the cholinergic projection neurons from the basal forebrain nuclei are mainly concentrated in the light fraction of synaptosomes and pre-synapses of the cholinergic interneurons in the heavy synaptosome fractions (cat, rat) [51-54].
1ChAT activity is expressed as a percentage relative to control (100%) in the synaptic membrane (sm, membrane-bound ChAT) and synaptoplasm (sp, water-soluble ChAT) sub-fractions of light and heavy synaptosomes isolated from the caudal brainstem and cortex of the intact, high-resistant and low-resistant HBH rats. Preparative procedures of subsynaptic fractions isolation and radiometric method of ChAT activity assay were described in detail [54].
In the Table, data are presented only in the synaptic sub-fractions in which there were significant changes in enzyme activity under HBH conditions to demonstrate individual response of the synaptic cholinergic pools to HBH in the intact, high- and low-resistant rat groups. *P< 0.05 and **P< 0.025 compared to the respective control, the Fisherâ¬?¬?s Exact test. ## r< 0.02 between sub-synaptic factions within brain structure, in which HBH initiated the conjugate changes in ChAT activity, the Pearsonâ¬?¬?s correlative test. Positive value of r-criterion, a positive correlation; negative value r-criterion, a negative correlation. Detailed analysis of the cholinergic reactions in these brain structures look in the earlier publication [37]. A summary of this analysis and results of correlation analysis between the ChAT activity in caudal brainstem and cortex sub-synaptic factions see in the text.
Thus, we conclude that the cortical cholinergic interneurons were involved in the HBH-initiated adaptation mechanisms in the brain of intact rats and the cholinergic cortical projection neurons in the brain of high-resistant rats.
Caudal brainstem
Caudal brainstem structures include several cholinergic sources, 1) projections from the reticular formation of the midbrain tegmentum [49,55-57]. 2) afferents of the nodose ganglion sensory neurons from the lung mechanoreceptors to the NTS [17,58,59], and 3) neurons of the pons Varolii and medulla oblongata, including neurons of reticular areas, NTS, and efferent parasympathetic preganglionic neurons of the motor cranial nerves nuclei [13,49,57,59-61].
Such phenotypic diversity of the cholinergic sources greatly complicates the identification of cholinergic pre-synapses in the synaptosomal fractions of this brain area. Nevertheless, a comparison of the existing data allowed making some assumptions. Thus, we analysed the possible medullary areas of targets for MEC and MLA in both experimental protocols of the hypoxic exposures.
Identification of cholinergic neuronal populations and pathways of caudal brainstem involved in the HBH preconditioning mechanisms and/or targets of their synaptic action
Systemic reaction to hypoxia, general information
It has been shown that the primary and immediate response to hypoxia of any severity involves activation of the autonomic sympathoexcitatory reticulospinal pathways, primarily from the peripheral or central chemoreceptors and the stimulation of respiration, heart activity and blood circulation aimed at restoring the blood level of O2 and CO2 exchange and pH [12,62-66]. The sequence and development of hypoxia-induced events are recounted [64,65]. In total, this systemic response occurs as a result of the wide cooperation of different functional groups of neurons of the autonomic respiratory and cardiovascular systems: sensory, primary and secondary chemoand barosensors of the nucleus tractus solitary (NTS) and ventrolateral medulla (VLM), relay neurons, reticulospinal neurons (area C1 of the rostral VLM) and preganglious parasympathetic (nuclei of the cranial nerves) and spinal motoneurons.
Another important adaptive express response to hypoxia is nonsympathetic activation of the cerebral blood flow that is based on the redistribution of blood flow towards the brain [65,67-70]. To date, two centres have been detected: the “parasympathetic cerebrovasodilator centre” or “dorsal facial area” (DFA) and the “medullary cerebral vasodilator area” (MCVA), both located in rostrolateral part of the medulla oblongata and DFA also in adjacent region of the pons varolii, the innervation of which, including hypoxia, initiates elevation of the cerebral blood flow by parasympathetic [22,67,71] or relay cerebripetal pathways [15,68].
In the study of the cerebripetal network, the connection of MCVA with the sympathetic vasomotor mechanisms is shown: in the sympathoexcitatory zone C1, the presence of the O2-sensitive neurons, activation of which excites the cerebripetal pathway; dependence of the cerebrovascular MCVA efficiency of safety of the reticulospinal pathway; and existence of collaterals from the sympathoexcitatory neurons in the cerebripetal direction [16,68].
All of these systemic reactions are revealed in cats [72] and respiratory reactions from those in rats [70,73,74] with high, intermediate and low resistance to very severe hypoxia (3% O2) with differences between these groups mainly in expression and duration of the responses before apnea. Under moderate hypoxia, the all compensatory reactions, including cerebral blood flow elevation, are maintained during the whole session of hypoxic training in rats [70,75], which is the physiological basis of hypoxic preconditioning [76-78].
Cholinergic pre-synapses of the caudal brainstem heavy synaptosomal fraction. Low-resistant rats
According to present data, the cholinergic response in the presynapses of the heavy synaptosomal fraction of the low-resistant rats corresponded to the functional characterisation of subtypes of the lung barosensitive C-fibres conducting afferentation to NTS through the nodose ganglion [79,80]. The existence of cholinergic neurons in this ganglion [81] and cholinergic fibres from nodose ganglion to NTS is shown by indirect [82] and then direct methods immunocytochemically [58]. Activation of C-fibre and/or rapidly adaptable airway receptors by histamine induces the bronchoconstriction and, in NTS, the activation of the barosensitive secondary neurons and expression of alpha3 nAChR subunit in these neurons [17]. The latter fact testifies to the cholinergic nature of this sensory signal.
It is known that apnea is often preceded by the classic reflex of Cfibres (frequent shallow breathing, bradycardia and hypotension) [80,83,84]. In the low-resistant rats, pronounced activation of ChAT, represented mainly by water-soluble ChAT activity [37,53], is observed at the time of apnea in the fraction of heavy synaptosomes, isolated from NTS [53] and, conversely, the inhibition of water-soluble ChAT in response to HBH was seen in the synaptoplasm sub-fraction of the caudal brainstem heavy synaptosomes.
Taken together, it seems that the reaction of C-fibre terminals was observed in the low-resistant rats. From all of these data, it could be assumed that the inhibition of ACh synthesis and non-quantum leakage in the pulmonary C-fibres might contribute to the inhibition of parasympathetic reflexes and/or extension of the time before the apnea. Therefore, we analysed the possibility of a link between the reaction of supposed C-fibres and nAChRs in NTS.
In NTS, nAChRs of alpha7 and non alpha7 subtypes have been identified [17,32,85,86]. Primary cardiac and pulmonary afferents, including cholinergic, are distributed rostrocaudally in the NTS and are focused along the intermedial line [13,82,87]. The cholinergic Cfibres enter the commissural and gelatinous subnuclei and presumably the medial subnucleus of the NTS [17,58,85]. The microinjection of nicotine into the medial NTS subnucleus enhances the main (glutamatergic) barosensitivity in an MEC-dependent manner [85] and microinjection into the commissural NTS subnucleus activates the bronchoconstriction through the alpha3 nAChR subunit and does not affect the vasomotor function [17]. In another study, microinjections of nicotine into NTS against the background selective nAChR blockers reveal the participation of alpha7 and alpha4beta2 nAChRs in the parasympathetic reactions of blood pressure decreases and bradycardia; the contribution of the first predominates over the latter and both reactions are not dependent on glutamatergic parasympathetic afferents [86]. Added to this, in the medial and commissural NTS subnuclei, the existence of neurons was revealed, on which the pulmonary C-fibres and afferents from cardiac receptors and peripheral chemoreceptors are converged [88,89]. Their costimulation also causes the bradycardia and suppression of respiratory activity.
NAChRs were detected on the catecholaminergic neurons of NTS as well as throughout the caudal brainstem [18,32]. The catecholaminergic neurons express all subunits of nAChRs in all NTS subnuclei but alpha7 receptor expression is restricted to the ventral region of NTS and alpha3 and beta4 subunit expression predominates in the dorsal region [32]. Data showing that the catecholaminergic neurons of all brainstem groups are involved in the network of vasopressin regulation of cardiovascular functions through neurosecretory vasopressin-synthesising cells in the paraventricular nucleus of the hypothalamus have been accumulated. It proved that the projections into this nucleus from NTS are noradrenergic (group A2) [18]. Vasopressin and agonists of alpha7, alpha4 beta2 and presumably alpha3 beta4 nAChRs provide sympatoactivation under systemic and central (IC) administration [18].
However, nicotine microinjections into NTS stimulate the parasympathetic response of a lowering of blood pressure and bradycardia side by side with the norepinephrine release into the paraventricular nucleus [18]. Recently, it was found that nicotine acts on vasopressin-stimulating neurons of NTS indirectly through presynaptic glutamate release [34]. These data bring us to the above report of the glutamate-mediated stimulation of the baroreflex by nicotine [85]. It has also been noted that the topography of cholinergic neurons in NTS coincides with the distribution of both noradrenergic neurons and sensory afferents, including the cholinergic from the nodose ganglion. This suggests that the noradrenergic and cholinergic neurons are in close association with the barosensitive neurons of NTS and some barosensitive neurons may be cholinergic [13,60,82].
From this, it follows that nAChRs of NTS are involved, apparently, in parasympathetic functions only. It is possible that the cholinergic Cfibres can act on nAChRs directly and indirectly affecting theirs through secondary cholinergic barosensitive neurons. Consistent with these data, in the low-resistant rats, MLA and MEC initiated the suppression of parasympathetic reflexes occurring in NTS and thereby increased their resistance to SHBH. With respect to MEC, our assumption is reinforced by data obtained by MEC microinjections into the intermedia and commissural subnuclei of NTS [23]. In the case of microinjections into intermedia subnucleus, MEC removes the inhibitory effect of ACh on the activity of the sympathetic and phrenic nerves. In the case of microinjections into the commissural subnucleus, MEC reduces experimentally-induced tachypnoea, i.e., rapid shallow breathing, which can develop at SHBH due to the accumulation of CO2.
Also, it is logical to assume that these effects of MLA and MEC could not appear after HBH, against the background of the reduced cholinergic C-fibres influences. In other words, HBH forestalled the protective effect of both antagonists.
Areas of MLA action on the HBH preconditioning or alpha7 nAChRs participation in the HBH preconditioning mechanisms. Low-resistant rats
Based on the above, it can be suggested that the suppression of resistance to hypoxia of the low-resistant rats under HBH by MLA took place in brainstem areas outside NTS, in which preconditioning effects could be stimulated through alpha7 nAChRs. That site may be sympathoexcitatory pressor zone C1 in the rostral VLM, located in the nucleus reticularis rostroventrolateralis. The accumulation of ACh in this area intensifies the spontaneous activity of reticulospinal (adrenergic) neurons, stimulates corresponding preganglionic sympathetic neurons of the spinal cord and increases the blood pressure and heart rate [90]. The rostral and caudal VLM catecholaminergic neurons express both alpha7 and not alpha7 nAChRs [32,91]. It was shown that: 1) adrenergic neurons from C1 zone send projections into the paraventricular nucleus; 2) the release of vasopressin in the zone C1 increases blood pressure; and 3) microinjection of nicotine in the rostral, but not the caudal VLM fully simulates the vasopressin-induced rise of the blood pressure [18]. In addition, the expression of alpha7 receptors and also alpha4 and alpha5 subunits of nAChR predominates in neurons of the C1 zone compared with the caudal VLM [32] and IC injection of MLA causes sympathoinhibition in relatively low dose of 0.1 μmol/kg, but not in a dose of 0.5 μmol/kg [19].
At the same time, it was found that direct cholinergic synaptic contacts on adrenergic neurons in the C1 zone are very few (8%) [92]. Therefore, it is assumed that the main way for direct cholinergic transmission on the adrenergic neurons is volume. The basis for this assumption was an especially high density of typical ChATCitation immunoreactive perisomatic patterns of small punctate terminal-like varicosities upon large and giant reticular neurons in the rostral VLM, including the Cl area and its surround, in rats, cats and hamsters [57,90].
The next site of possible alpha7 nAChR preconditioning influences is located in the rostrolateral medulla. Dorsolateral towards the rostral part of the nucleus facialis, a group of neurons was identified whose stimulation reduces the cerebral vascular resistance and increases the rate of cerebral blood flow known as the “parasympathetic cerebrovasodilator centre” in rats [67] or DFA in cats [22,71]. Local microinjections of ACh into DFA dose-dependently increase the synaptic release of glutamate and/or nitric oxide and the rate of cerebral blood flow without affecting the mean arterial pressure and heart rate. In these cholinergic effects, nAChRs are involved, predominantly alpha7 subtype, but not mAChRs. The presence of these nAChRs in DFA is assumed on the nitrergic and/or glutamatergic fibres which stimulate the preganglionic neurons of the parasympathetic 7th and 9th cranial nerves for increasing cerebral blood flow.
Another bulbar centre of the regulation of cerebral blood flow, MCVA, consists of the cerebripetal neurons of not morphologically specified region of the rostral VLM near zone C1. Microinjections of nicotine into MCVA, like hypoxia, raise the cerebral blood flow [15]. However, a characteristic property of this region in rats is sensitivity to severe hypoxia from arterial O2 pressure 40 mm Hg (from hypoxia<7% O2) and insensitivity to moderate hypoxia in the range of 70-90 mm Hg (10-15% O2), at least for a short time of exposure (2-2.5 min) [68].
The sources of the cholinergic cerebrovascular influences in DFA and MCVA are not identified, as well as in the C1 zone.
Then, it is possible that alpha7 nAChRs participated in the preconditioning effects of respiratory neurons. ACh potentiates inspiratory activation of the motoneurons in the hypoglossal nucleus of the 12th cranial nerve when acting on nAChRs post-synaptically [20,93].
Cholinergic pre-synapses of the caudal brainstem light synaptosomal fraction. High-resistant rats
In the high-resistant rats, ChAT activity does not change at the time of apnea in any of the synaptosomal fractions of NTS [53]. On the other hand, HBH provoked inhibition of the water-soluble ChAT activity in the light synaptosomal synaptoplasm sub-fraction of the caudal brainstem. According to this data, localization of the nerve endings of the sensitive to hypoxia cholinergic neurons in this group of rats may be outside NTS. These characteristics correspond to the cholinergic reticular interneurons of the caudal brainstem, the projective neurons and, possibly, neurons of NTS. About the latter, their cholinergic terminals within NTS are not detected [60].
It was revealed immunocytochemically that ChAT-positive axons penetrate all areas of the medulla oblongata, including the NTS and VLM [20,49,57,90,94]. Also, the sites in NTS, VLM and some other medullary areas are revealed to form more compact groups of ChATpositive terminals [90].
In our study, the additional result was given about correlation between the water-soluble ChAT activity in the caudal brainstem and membrane-bound ChAT activity in the cortical projection neurons selectively in the high-resistant rats. This allowed us to assume that the inhibition of the water-soluble ChAT activity under HBH conditions in this group of rats occurred in pre-synapses of the projection neurons from laterodorsal (LDT) and/or pedunculopontine (PPT) tegmental cholinergic nuclei of the middle brain. LDT and more intensively PPT send plurality of the fibres to both the pontine and medulla oblongata nuclei and also to the higher brain structures, including the basal forebrain nuclei [56,57,95]. The density of the projection fibres of these nuclei is considerably greater than the fibre network of the cholinergic interneurons in the reticular formation of the medulla oblongata [49,56,57]. At the same time, representation of these projections in the NTS is minimal or absent [55].
The cholinergic neurons of the same two tegmental nuclei are functionally closely associated with the cortical cholinergic projection neurons of the basal forebrain nuclei. The brainstem send most of their projections to the forebrain nuclei from LDT and PPT and very few from the medulla oblongata [95]. The authors estimated that 21% of the identified cholinergic neurons of the basal ganglia, which project their fibres to the cortex, are activated or inhibited following stimulation of the cholinergic neurons from LDT and PPT.
Also, some neurons in these tegmental nuclei send the projections to the medulla oblongata as well as to the basal ganglia [56]. The cholinergic neurons of LDT and PPT are the main switch between the cortical cholinergic projective neurons and brainstem formations. It is important to note that the middle brain projective neurons of the basal forebrain are different from the cortical projective neurons topographically, by their electrophysiological properties and they are non-cholinergic. Only a few cholinergic projective cortical neurons send their collaterals to the brainstem too [96]. For this reason, the reaction of the direct cholinergic projections from the forebrain among the total cholinergic synaptic pool of the brainstem is not possible to identify easily by our biochemical methods.
From the above data, it also follows that in the conjunction tegmentum-cortex, the inhibition of membrane-bound ChAT activity (and ACh quantum releasing) in the cortical projective cholinergic neurons was a consequence of the afferent influences from the tegmental PPT/LDT nuclei, and not vice versa, whereas feedback between these neuronal groups has, at least, one relay neuron. Such indirect connections, as a rule, do not achieve statistical significance in biological systems. Therefore, we believe that this adaptive inhibition of the cortical neuronal projections was caused by neuronal influences and not by tissue oxygen deficiency in the cortex. Also, a coordinated response to HBH of these two cholinergic neuronal populations might reflect the repair processes, because HBH restores the resistance of the high-resistant rats which is chronically reduced in this group after the initial pre-testing SHBH exposure [36].
Neurons of the PPT and LDT nuclei are projected into many areas of the caudal brainstem [56]. The targets of some tegmental cholinergic projections are known. We shall describe the cholinergic effects on the identified sites, in which the functional inhibition would contribute to the hypoxic preconditioning to put off apnea.
Already mentioned motoneurons of the upper airway from the hypoglossal 12th cranial nerve nucleus are located in the dorsal part of the medulla oblongata. The hypoglossal motoneurons receive permanent excitation drive from the inspiratory, mainly glutamatergic, neurons from the medullary caudal intermediate reticular region. Intensification of inspiratory activation, for example, in OSA patients (obstructive apnea during sleep), increases the innervation of these motoneurons and, respectively, of the upper airways raises their tone and thus prevents their collapse. The activity of the motoneurons is modulated by cholinergic neurons as the reticular from the same medullary zone and the projective from PPT and LDT. Cholinergic neurons of both populations have direct links with the hypoglossal motoneurons and the projective tegmental neurons have additionally indirect links through the neurons of the pontine dorsomedial reticular formation [20]. The hypoglossal motoneurons are postsynaptically activated by nAChRs stimulation [93] and are presynaptically inhibited by the M2 subtype of muscarinic cholinoreceptors (mAChR) stimulation [97].
According to our data, nAChR antagonists did not affect the action of HBH in the high-resistant rats at our low doses. This leads us to conclude that the nAChRs of the high-resistant rats were not involved in the preconditioning mechanisms. On the other hand, attenuation of the non-quantum leakage of ACh could weaken presynaptic inhibition of the hypoglossal motoneurons through mAChRs.
Another target of the cholinergic PPT and LDT projections is different types of the laryngeal motoneurons of the upper airway located in the loose formation of the nucleus ambiguous in the rostral VLM and innervating the dilator or constrictor laryngeal muscles during, respectively, inspiration or expiration [21]. In this site, mAChRs were identified involved in the modulation of the activity of the intrinsic muscles of the larynx. The author, used cholinergic and synaptic markers, showing that 1) at least 90% of the cholinergic information to the laryngeal motoneurons is transmitted through synapses, 2) the cholinergic synapses predominate on the cell soma and proximal dendrites for both inspiratory and expiratory modulated laryngeal motoneurons which expects the powerful influence of cholinergic inputs on the activity of respiratory modulated laryngeal motoneurons, 3) expiratory modulated motoneurons receive a significantly greater number of the synaptic inputs than inspiratory ones, and 4) cholinergic synapses on the laryngeal motoneurons have relatively small sizes that correspond to the sizes of synaptosomes of the light fraction [52]. According to other researchers, the majority of laryngeal motoneurons respond to the iontophoretic application of ACh with muscarinic receptor-mediated depolarisation [14]. Taken together, it is likely that the decline of expiratory influences on this site might exercise bulbar preconditioning action in the high-resistant rats.
The third identified site of cholinergic PPT and LDT influence is located in the caudal VLM [16]. Under cholinergic impacts in this area, the suppression of cardiovascular functions was developed: drop in blood pressure and heart rate, bradycardia. However, these effects depended on nAChRs only. They were initiated by microinjections into the caudal VLM of nicotine and completely blocked by nAChR antagonists of the alpha7 subtype alpha-bungarotoxin and non-alpha7 subtype MEC.
Intact rats
In the intact rats, HBH provoked an increase in the water-soluble ChAT activity in the same light synaptosomal synaptoplasm subfraction of the caudal brainstem, in which the water-soluble enzyme activity was inhibited in the high-resistant rats. Simultaneously, the water-soluble ChAT was activated in the cortical interneurons (the heavy synaptosomal synaptoplasm sub-fraction).
There was no effect of the nAChR antagonists for resistance to hypoxia in the both experimental protocols. Therefore, cholinergic response, revealed in the caudal brainstem of the intact rats, did not provide any help for the identification of neuronal population reacted to HBH. Nevertheless, the oppositely directed ChAT reaction in the same synaptosomal fraction to achieve the same physiological effect indicates different preconditioning mechanisms in the intact and highresistant rats. This is also indicated the cholinergic response to HBH in the cortex in these two groups of rats.
There was no correlation between ChAT activity in the caudal brainstem and in the cortex in this rat group because of the absence of a direct link between the cholinergic brainstem neurons and cortical interneurons. ACh synthesis activation under HBH in the cortical interneurons could be related to their function of redistribution of the blood flow towards the brain. With respect to cerebral vessels, direct contacts with small cortical vessels and vasodilator effects of both the cholinergic projective neurons and interneurons were detected [98-100]. Recall that under moderate hypoxia, cerebral blood flow elevation is maintained during the whole session of hypoxic training [70,75,76].
Thus in intact brain, the cortical cholinergic interneurons might be involved in the local mechanisms to maintain of cerebral blood flow. In contrast, inhibition of the cholinergic influences of projection neurons in the cortex of the high-resistant rats was apparently the result of their functional relationship with brainstem and as a consequence could be involved in other adaptive or reparative reactions.
Conclusion
Neuronal, mediator and receptor mechanisms of hypoxic preconditioning are poorly understood to date. In the normal physiological conditions, the central cholinergic influences do not play a critical role in the autonomic regulation of respiratory or cardiovascular activity like glutamatergic, adrenergic or GABAergic systems [7,17,62,85,101,102], but perform a modulatory role. On the other hand it is possible that the modulatory systems are the key in the preconditioning mechanisms.
From our results, the cholinergic system of the caudal brainstem was involved in the adaptive mechanisms in all experimental rat groups. Cholinergic responses to HBH differed from each other in the rats with different innate resistance to severe hypoxia and prior hypoxic experience. At the same time, the same hypoxic preconditioning effect is revealed in these rat groups. Using the literature and own data, we have tried to identify some cholinergic neuronal populations or areas of their actions which may be involved in the hypoxic preconditioning mechanisms to put off apnea.
As we showed in this Review and earlier [37], the single pre-testing under SHBH altered synaptic and neuronal preconditioning mechanisms. This is consistent with the observations in respect of the effects of the cholinergic C-fibres [17] and cholinergic projections from midbrain PPT and LDT nuclei into the nucleus of the hypoglossal nerve [20], the reactivation of which, according to our assumption, manifested itself in the brain of low- and high-resistant rats, respectively. The authors of both studies suggest that these mechanisms may not play a significant role in the natural physiological conditions but can be important in certain impacts and loadings on an organism.
In the high-resistant rat group, the cortical cholinergic projections from the forebrain nuclei functionally related to tegmental cholinergic projections and, we believe, their adaptive inhibition is regulated by autonomic cardiorespiratory systems of the caudal brainstem.
In the intact rat group, cholinergic response to HBH was detected in the cortical pre-synapses, which most likely belongs to the cortical interneurons. These data indicate the possible existence of the local mechanisms of hypoxic preconditioning in the cortex of intact brain that are independent of the autonomic brainstem systems.
Unfortunately, we have not obtained any information to identify the populations of cholinergic neurons of the caudal brainstem, involved in the hypoxic preconditioning mechanisms in the intact rats. In our previous study, we have substantiated that reaction to HBH in the intact rats can be based on different preconditioning mechanisms and that it is necessary to look for non-stressor methods for the separation of animals in their sensitivity to adaptive hypoxia [36]. The separation of the intact rats will help to reveal innate neuronal mechanisms to put off apnea that can be hidden in their complex.
At the same time, we believe that the variety of pathways in the regulation of vital functions such as breathing and blood circulation is not only the result of their individual organization. The multiplicity of pathways must be inherent to every organism as the ability to make a quick choice to maintain its viability.
The variety of neuronal pathways to achieve the same physiological affect demonstrates a great adaptive potential of brain, and the specific mechanisms of its realization may be a promising therapeutic targets.
Acknowledgement
We are grateful to the direction of Institute of General Pathology and Pathophysiology that supported and funded our research. And we would like to thank the team of the Proof-Reading-Service.com and personally Louise Bourdon for editing and proofreading the English of our manuscript. Also we would like to thank of our colleague Kopaladze RA for technical assistance.
References
- Hawaleshka A, Jacobsohn E (1998) Ischaemic preconditioning: Mechanisms and potential clinical applications. Can J Anaesth 45: 670-682.
- Park HK, Seol IJ, Kim KS (2011) Protective effect of hypoxic preconditioning on hypoxic-ischemic injured new-born rats. J Korean Med Sci 26: 1495-1500.
- Speer R. Ratan RR (2016) Hypoxic adaptation in the nervous system: Promise for novel therapeutics for acute and chronic neurodegeneration. Adv Exp Med Biol 903: 221-243.
- Overath JM, Gauer S, Obermüller N, Schubert R, Schäfer R, et al. (2016) Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp Cell Res 342: 175-183.
- Araki T, Kato H, Fujiwara T, Kogure K, Itoyama Y (1995) Alteration of [3H]hemicholinium-3 binding in the post-ischaemic gerbil brain. Neuroreport 6: 561-564.
- Saether K, Hilaire G, Monteau R (1987) Dorsal and ventral respiratory groups of neurons in the medulla of the rat. Brain Res 419: 87-96.
- Tarakanov IA, Safonov VA (2003) Neurohumoral concept of central respiratory regulation. Pathogenesis 1: 11-24.
- Richter DW, Smith JC (2014) Respiratory rhythm generation in vivo. Physiology 29: 58-71.
- Mitchell RA, Berger AJ (1975) Neural regulation of respiration. Am Rev Respir Dis 111: 206-224.
- Edlow BL, McNab JA, Witzel T, Kinney HC (2016) The structural connectome of the human central homeostatic network. Brain Connect 6: 187-200.
- Feldberg W, Guertzenstein PG (1972) A vasodepressor effect of pentobarbitone sodium. J Physiol 224: 83-103.
- Loeschcke HH (1982) Central chemosensitivity and the reaction theory. J Physiol 332: 1-24.
- Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ (1990) Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol 292: 1-53.
- Haji A, Furuichi S, Takeda R (1996) Effects of iontophoretically applied acetylcholine on membrane potential and synaptic activity of bulbar respiratory neurones in decerebrate cats. Neuropharmacology 35: 195–203.
- Golanov EV, Ruggiero DA, Reis DJ (2000) A brainstem area mediating cerebrovascular and EEG responses to hypoxic excitation of rostral ventrolateral medulla in rat. J Physiol 529: 413-429.
- Aberger K, Chitravanshi VC, Sapru HN (2001) Cardiovascular responses to microinjections of nicotine into the caudal ventrolateral medulla of the rat. Brain Res 892: 138-146.
- Haxhiu MA, Kc P, Moore CT, Acquah SS, Wilson CG, et al. (2005) Brain stem excitatory and inhibitory signaling pathways regulating bronchoconstrictive responses. J Appl Physiol 98: 1961-1982.
- Moore C, Wang Y, Ramage AG (2008) Cardiovascular effects of activation of central alpha7 and alpha4beta2 nAChRs: A role for vasopressin in anaesthetized rats. Br J Pharmacol 153: 1728-1738.
- Moore C, Wang Y, Ramage AG (2011) Nicotine's central cardiovascular actions: Receptor subtypes involved and their possible physiological role in anaesthetized rats. Eur J Pharmacol 668: 177-183.
- Volgin DV, Rukhadze I, Kubin L (2008) Hypoglossal premotor neurons of the intermediate medullary reticular region express cholinergic markers. J Appl Physiol 105: 1576-1584.
- Bautista TG, Sun QJ, Zhao WJ, Pilowsky PM (2010) Cholinergic inputs to laryngeal motoneurons functionally identified in vivo in rat: A combined electrophysiological and microscopic study. J Comp Neurol 518: 4903-4916.
- Gong CL, Leung YM, Huang YP, Lin NN, Hung YW, et al. (2010) Nicotine activation of neuronal nitric oxide synthase and guanylyl cyclase in the medulla increases blood flow of the common carotid artery in cats. Neurosci Lett 486: 122-126.
- Furuya WI, Bassi M, Menani JV, Colombari E, Zoccal DB, et al. (2014) Differential modulation of sympathetic and respiratory activities by cholinergic mechanisms in the nucleus of the solitary tract in rats. Exp Physiol 99: 743-758.
- Takada-Takatori Y, Kume T, Izumi Y, Ohgi Y, Niidome T, et al. (2009) Roles of nicotinic receptors in acetylcholinesterase inhibitor-induced neuroprotection and nicotinic receptor up-regulation. Biol Pharm Bull 32: 318-324.
- van Rensburg R, Errington DR, Ennaceur A, Lees G, Obrenovitch TP, et al. (2009) A new model for the study of high-K(+)-induced preconditioning in cultured neurones: Role of N-methyl-d-aspartate and alpha7-nicotinic acetylcholine receptors. J Neurosci Methods 177: 311-316.
- Liu AJ, Zang P, Guo JM, Wang W, Dong WZ, et al. (2012) Involvement of acetylcholine-α7nAChR in the protective effects of arterial baroreflex against ischemic stroke. CNS Neurosci Ther 18: 918-926.
- Xiong J, Yuan YJ, Xue FS, Wang Q, Cheng Y, et al. (2012) Postconditioning with α7nAChR agonist attenuates systemic inflammatory response to myocardial ischemia-reperfusion injury in rats. J Cardiovasc Pharmacol 59: 507-513.
- Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47: 699-729.
- He L, Dinger B, Fidone S (2005) Effect of chronic hypoxia on cholinergic chemotransmission in rat carotid body. J Appl Physiol 98: 614-619.
- Dehkordi O, Kc P, Balan KV, Haxhiu MA (2006) Airway-related vagal preganglionic neurons express multiple nicotinic acetylcholine receptor subunits. Auton Neurosci 128: 53-63.
- Eguchi S, Miyashita S, Kitamura Y, Kawasaki H (2007) Alpha3beta4-nicotinic receptors mediate adrenergic nerve- and peptidergic (CGRP) nerve-dependent vasodilation induced by nicotine in rat mesenderic arteries. Br J Pharmacol 151: 1216-1223.
- O'Leary KT, Loughlin SE, Chen Y, Leslie FM (2008) Nicotinic acetylcholine receptor subunit mRNA expression in adult and developing rat medullary catecholamine neurons. J Comp Neurol 510: 655-672.
- Shao XM, Feldman JL (2009) Central cholinergic regulation of respiration: Nicotinic receptors. Acta Pharmacol Sin 30: 761-770.
- Feng L, Uteshev VV (2014) Projection target-specific action of nicotine in the caudal nucleus of the solitary tract. J Neurosci Res 92: 1560-1572
- Zakharova EI, Dudchenko AM, Germanova EL (2011) Effects of preconditioning on the resistance to acute hypobaric hypoxia and their correction with selective antagonists of nicotinic receptors. Bull Exp Biol Med 151: 179-182.
- http: //file.scirp.org/pdf/JBiSE_2016112214300822.pdf
- Zakharova EI, Germanova EL, Kopaladze RA, Dudchenko AM (2013) Central cholinergic systems in the mechanisms of hypoxic preconditioning: Diverse pathways of synaptic reorganization in vivo. Neurochemical J 7: 45–55.
- Lukyanova LD, Germanova EL, Tsibina TA, Kopaladze RA, Dudchenko A (2008) Efficiency and mechanism for different regimens of hypoxic training: The possibility of optimization of hypoxic therapy. Pathogenez 6: 32-36.
- Davies AR, Hardick DJ, Blagbrough IS, Potter BV, Wolstenholme AJ, et al. (1999) Characterisation of the binding of [3H]methyllycaconitine: A new radio ligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology 38: 679-690.
- Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, et al. (2003) Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci 92: 1051-1057.
- Stegelmeier BL, Hall JO, Gardner DR, Panter KE (2003) The toxicity and kinetics of larkspur alkaloid, methyllycaconitine, in mice. J Anim Sci 81: 1237-1241.
- Nickell JR, Grinevich VP, Siripurapu KB, Smith AM, Dwoskin LP (2013) Potential therapeutic uses of mecamylamine and its stereoisomers. Pharmacol Biochem Behav 108: 28-43.
- Kobzar AI (2006) Applied mathematical statistics for engineers and scientists. Physmatlit: 816.
- Macallan DR, Lunt GG, Wonnacott S, Swanson KL, Rapoport H, et al. (1988) Methyllycaconitine and (+)-anatoxin-a differentiate between nicotinic receptors in vertebrate and invertebrate nervous systems. FEBS Lett 226: 357-363.
- Young JM, Shytle RD, Sanberg PR, George TP (2001) Mecamylamine: New therapeutic uses and toxicity/risk profile. Clin Ther 23: 532-565.
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI (1983) Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10: 1185-1201.
- Matthews DA, Salvaterra PM, Crawford GD, Houser CR, Vaughn JE (1987) An immunocytochemical study of choline acetyltransferase-containing neurons and axon terminals in normal and partially deafferented hippocampal formation. Brain Res 402: 30-43.
- Eckenstein FP, Baughman RW, Quinn J (1988) An anatomical study of cholinergic innervations in rat cerebral cortex. Neuroscience 25: 457-474.
- Woolf NJ (1991) Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37: 475-524.
- Frotscher M, Vida I, Bender R (2000) Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience 96: 27-31.
- Mukhin EI, Zakharova EI, Kikteva EA (2002) Comparison of the cholinergic system in neocortical field Ep in cats with strong and weak cognitive abilities. Neurosci Behav Physiol 32: 379-387.
- Zakharova EI, Dudchenko AM, Svinov MM, Ivanov DS, Ignat’ev IV (2001) Comparative characteristic of the brain cholinergic systems in rats with low and high resistance to oxygen deficiency. Neirokhimiya 18: 119–131.
- Zakharova EI, Dudchenko AM, Svinov MM, Fedorova MM, Germanova EL (2010) Cholinergic systems of the rat brain and neuronal reorganization under conditions of acute hypoxia. Neurochemical J 4: 290–303.
- https: //www.hindawi.com/journals/ijad/2010/954589/
- Rye DB, Lee HJ, Saper CB, Wainer BH (1988) Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 269: 315–341.
- Woolf NJ, Butcher LL (1989) Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull 23: 519-540.
- Jones BE (1990) Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol 295: 485-514.
- Reynolds DJ, Lowenstein PR, Moorman JM, Grahame-Smith DG, Leslie RA (1994) Evidence for cholinergic vagal afferents and vagal presynaptic M1 receptors in the ferret. Neurochem Int 25: 455-464.
- Schäfer MK, Eiden LE, Weihe E (1998) Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. I. Central nervous system. Neuroscience 84: 331-359.
- Armstrong DM, Rotler A, Hersh LB, and Pickel VM (1988) Localization of choline acetyltransferase in perikarya and dendrites within the nuclei of the solitary tracts. J Neurosci Res 20: 279–290.
- Kc P, Mayer CA, Haxhiu MA (2004) Chemical profile of vagal preganglionic motor cells innervating the airways in ferrets: The absence of non-cholinergic neurons. J Appl Physiol 97: 1508-1517.
- Reis DJ, Ruggiero DA, Morrison SF (1989) The C1 area of the rostral ventrolateral medulla oblongata: A critical brainstem region for control of resting and reflex integration of arterial pressure. Am J Hypertens 2: 363S-374S.
- Richter D W, Bischoff A, Anders KM, Windhorst U (1991) Response of the medullary respiratory network of the cat to hypoxia. J Physiol 443: 231–256.
- Sun MK, Reis DJ (1994) Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol 44: 197-219.
- Thomas T, Marshall JM (1994) Interdependence of respiratory and cardiovascular changes induced by systemic hypoxia in the rat: The roles of adenosine. J Physiol 480: 627-636.
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM (1999) Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol 514: 567-578.
- Nakai M, Tamaki K, Ogata J, Matsui Y, Maeda M (1993) Parasympathetic cerebrovasodilator center of the facial nerve. Circ Res 72: 470-475.
- Golanov EV, Reis DJ (1996) Contribution of oxygen-sensitive neurons of the rostral ventrolateral medulla to hypoxic cerebral vasodilatation in the rat. J Physiol. 495: 201-216.
- Reis DJ, Golanov EV, Galea E, Feinstein DL (1997) Central neurogenic neuroprotection: Central neural systems that protect the brain from hypoxia and ischemia. Ann N Y Acad Sci 835: 168-186.
- Sanotskaya NV, Matsievskii DD, Lebedeva MA (2012) Acute hypoxia influence on pulmonary and systemic blood circulation. Patogenez 10: 56-59.
- Gong CL, Leung YM, Wang MR, Lin NN, Lee TJ, et al. (2013) Neurochemicals involved in medullary control of common carotid blood flow. Neuropharmacol 11: 513-520.
- Sanotskaia NV, Matsievskiń≠ DD, Tarakanov IA (1999) Changes in hemodynamics and respiration in animals with various resistance to acute hypoxia. Bull Exp Biol Med 128: 906-909.
- Sanotskaya NV, Matsievskii DD, Lebedeva MA (2004) Changes in hemodynamics and respiration in rats with different resistance to acute hypoxia. Bull Exp Biol Med 138: 18-22.
- Sanotskaya NV, Matsievskii DD, Lebedeva MA (2008) Effect of picrotoxin on organism's resistance to acute severe hypoxia. Bull Exp Biol Med 145: 177-180.
- Tabata M, Kurosawa H, Kikuchi Y, Hida W, Ogawa H, et al. (2001) Role of GABA within the nucleus tractus solitarii in the hypoxic ventilatory decline of awake rats. Am J Physiol Regul Integr Comp Physiol 281: R1411-R1419.
- Xing T, Fong AY, Bautista TG, Pilowsky PM (2013) Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin Exp Pharmacol Physiol 40: 602-609.
- Belchenko LA (2001) Adaptation of humans and animals to various kinds of hypoxia. Soros Educational Journal 7: 1-7.
- Chizhov AYa, Bludov AA (2004) Mechanisms and foundations of resonant hypoxitherapy in problems of hypoxia: Molecular, physiological, and medical aspects. Istoki, Voronezh.
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, et al. (2004) Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556: 905-917.
- Filippova LV, Nozdrachev AD (2013) An overview of the pulmonary sensory receptors. Usp Fiziol Nauk 44: 93-112.
- Palouzier B, Barrit-Chamoin MC, Portalier P, Ternaux JP (1987) Cholinergic neurons in the rat nodose ganglia. Neurosci Lett 80: 147-152.
- Helke CJ, Handelmann GE, Jacobowitz DM (1983) Choline acetyltransferase activity in the nucleus tractus solitarius: Regulation by the afferent vagus nerve. Brain Res Bull 10: 433-436.
- Bonham AC, Joad JP (1991) Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol 441: 95-112.
- Seifert E, Trippenbach T (1995) Baclofen attenuates cardiorespiratory effects of vagal C fiber stimulation in rats. Can J Physiol Pharmacol 73: 1485-1494.
- Ashworth-Preece M, Jarrott B, Lawrence AJ (1998) Nicotinic acetylcholine receptors in the rat and primate nucleus tractus solitarius and on rat and human inferior vagal (nodose) ganglia: Evidence from in vivo microdialysis and [125I] alpha-bungarotoxin autoradiography. Neuroscience 83: 1113-1122.
- Dhar S, Nagy F, McIntosh JM, Sapru HN (2000) Receptor subtypes mediating depressor responses to microinjections of nicotine into medial NTS of the rat. Am J Physiol Regul Integr Comp Physiol 279: R132-R140.
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR (2006) Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618-627.
- Paton JF (1998) Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365-2373.
- Silva-Carvalho L, Paton JF, Rocha I, Goldsmith GE, Spyer KM (1998) Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J Neurophysiol 79: 2374-2382.
- Giuliano R, Ruggiero DA, Morrison S, Ernsberger P, Reis DJ (1989) Cholinergic regulation of arterial pressure by the C1 area of the rostral ventrolateral medulla. J Neurosci 9: 923-942.
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, et al. (1989) Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: A hybridization histochemical study in the rat. J Comp Neurol 284: 314-335.
- Milner TA, Pickel VM, Morrison SF, Reis DJ (1989) Adrenergic neurons in the rostral ventrolateral medulla: Ultrastructure and synaptic relations with other transmitter-identified neurons. Prog Brain Res 81: 29-47.
- Chamberlin NL, Bocchiaro CM, Greene RW, Feldman JL (2002) Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience 115: 861–870.
- Holmes CJ, Mainville LS, Jones BE (1994) Distribution of cholinergic, GABAergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat. Neuroscience 62: 1155-1178.
- Semba K, Reiner PB, McGeer EG, Fibiger HC (1988) Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry and electrophysiology in the rat. J Comp Neurol 267: 433-453.
- Semba K, Reiner PB, McGeer EG, Fibiger HC (1989) Brainstem projecting neurons in the rat basal forebrain: Neurochemical, topographical and physiological distinctions from cortically projecting cholinergic neurons. Brain Res Bull 22: 501-509.
- Bellingham MC, Berger AJ (1996) Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol 76: 3758-3770.
- Dauphin F, Lacombe P, Sercombe R, Hamel E, Seylaz J (1991) Hypercapnia and stimulation of the substantia innominata increase rat frontal cortical blood flow by different cholinergic mechanisms. Brain Res 553: 75-83.
- Chédotal A, Cozzari C, Faure MP, Hartman B K, Hamel E (1994) Distinct choline acetyltransferase (ChAT) and vasoactive intestinal polypeptide (VIP) bipolar neurons project to local blood vessels in the rat cerebral cortex. Brain Res 646: 181-193.
- Vaucher E, Hamel E (1995) Cholinergic basal forebrain neurons project to cortical microvessels in the rat: Electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci 15: 7427-7441.
- Aicher SA, Milner TA, Pickel VM, Reis DJ (2000) Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull 51: 107-110.
- Shevtsova NA, Büsselberg D, Molkov YI, Bischoff AM, Smith JC, et al. (2014) Effects of glycinergic inhibition failure on respiratory rhythm and pattern generation. Prog Brain Res 209: 25-38.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 4802
- [From(publication date):
December-2016 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 3889
- PDF downloads : 913