Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
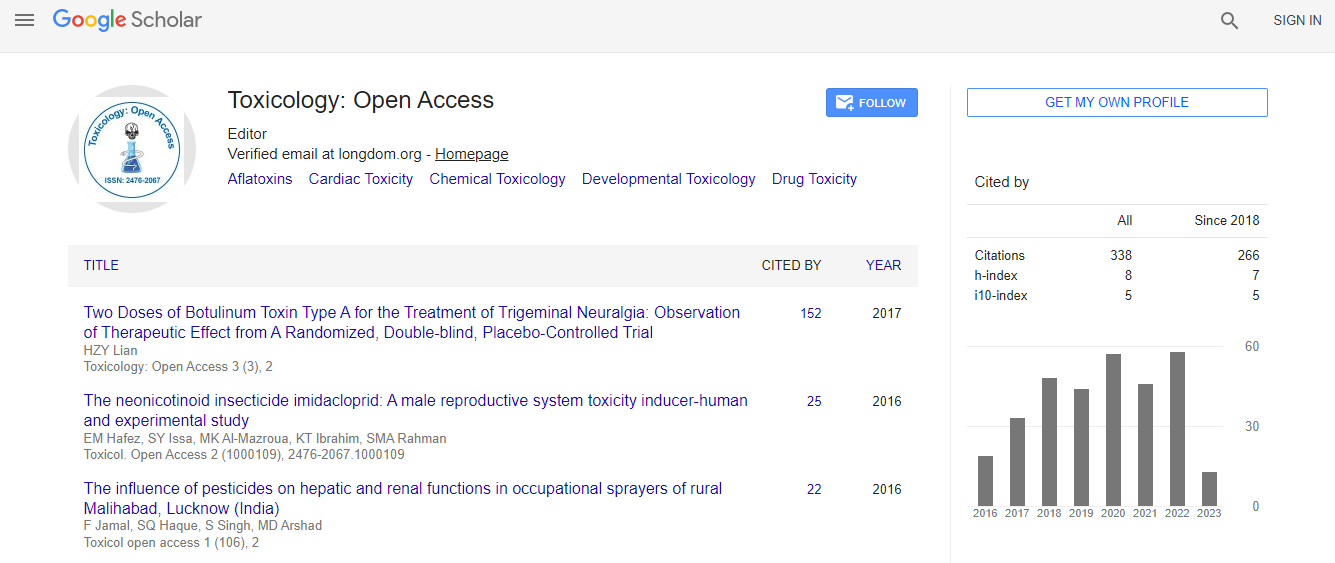
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Could diagnostic biomarkers be used to predict the response to biologic therapy in Rheumatoid Arthritis?
8th World Congress on Toxicology and Pharmacology
Gavrila B I, Claudia Ciofu, Carina Mihai, Bojinca M, Stoica V, Gabriela Udrea, Ciotaru D, Mihaela Surcel, Adrina Munteanu, Ursaciuc C and Eugenia Panaitescu
Carol Davila University of Medicine and Pharmacy, Romania INCD, Romania
ScientificTracks Abstracts: Toxicol Open Access
Abstract
Background: Biologic therapies have revolutionized the treatment of Rheumatoid Arthritis (RA). Despite these advances, 20-40% of the patients are declared nonresponders to at least one of the therapies. The patient exposure to the potential side effects and high costs requires the discovery of a biomarker that could identify those who can benefit from the pretreatment of a certain therapy. We proposed to test the predictive role for the response to biologic therapy of diagnostic biomarkers used in RA: rheumatoid factor (RF) isotypes IgM and IgA, anti-cyclic citrullinated peptide (anti-CCP) and auto-antibodies against mutated citrullinated vimentin (anti-MCV). We also followed the evolution of serum levels of these biomarkers under biologic therapy. Methods: Prospective and observational study including 64 patients followed 12 months with active RA, uncontrolled by conventional synthetic DMARDs or declared non-responders to one of the biologic DMARDs. Results: Lower baseline titres of RF type Ig M (51.36├?┬▒95.359 U/ml, p=0.01629), Ig A (22.45├?┬▒61.256 U/ml, p=0.03336) and anti-CCP (60.82├?┬▒26.331ng/ml, p=0.00011) had predictive value for achieving a good EULAR response at 6 months. Regarding anti-MCV baseline titres, there were no differences between groups at 6 months (p=0.45914) or at 12 months (p=0.11354). Grouping patients in 2 categories (responders/non-responders), we identified significant differences between groups only for anti-CCP and response at 6 months (responders 96.04├?┬▒50.355ng/ml, non-responders 146.16├?┬▒41.68ng/ml, p=0.02834). For the EULAR response at 12 months, lower baseline titres for RF type Ig M (92.93├?┬▒120.22 U/ml, p=0.01032) and Ig A (49.96├?┬▒98.08 U/ml, p=0.00247) had predictive value for achieving a good response at 12 months. We didn├ó┬?┬?t obtain other information grouping patients in 2 categories. Regarding the evolution of serum levels, we noticed a reduction for all four biomarkers tested, statistically significant at 6 and / or 12 months from baseline. Conclusion: Besides from their diagnostic role, these biomarkers could be used for other purposes in Rheumatoid Arthritis.Biography
Gavril├?┬? B I completed his PhD in 2016 from University of Medicine and Pharmacy, Bucharest (Romania). Currently, he is working as an Assistant Professor at Department of Internal Medicine and Rheumatology, Bucharest.
Email: bogdang135@yahoo.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi