Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
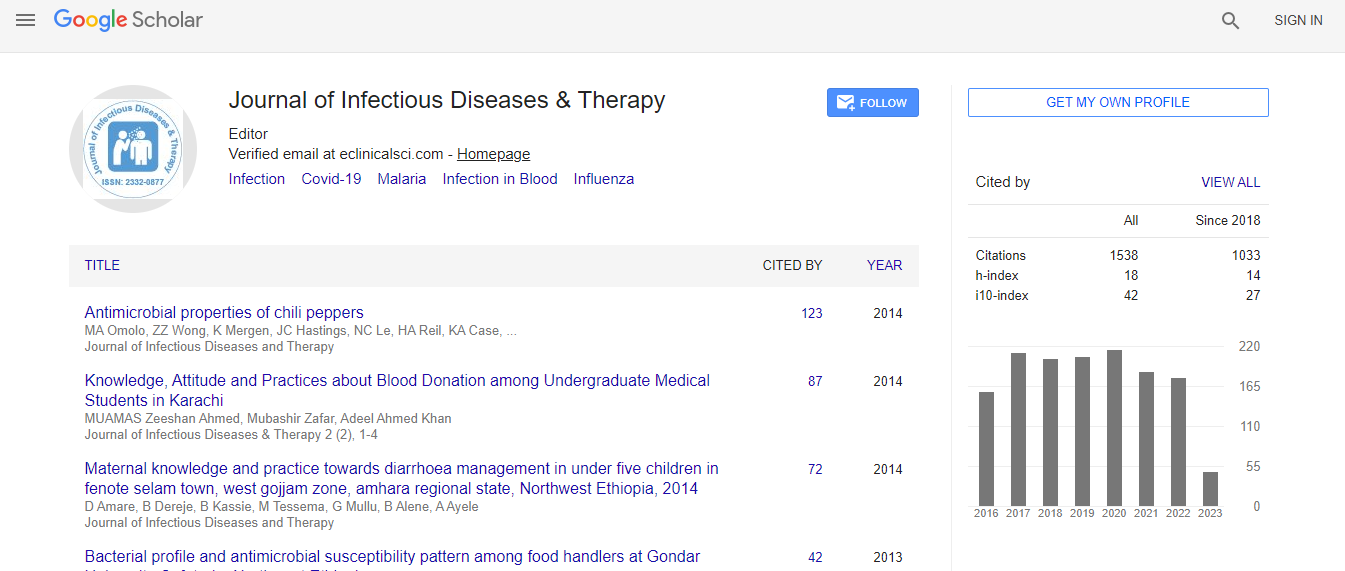
Google Scholar citation report
Citations : 1529
Journal of Infectious Diseases & Therapy received 1529 citations as per Google Scholar report
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Human properdin binds influenza A virus and restricts infection in a complement-independent manner
International Conference on Influenza
Lubna Kouser, Annapurna Nayak, Robert Sim, Uday Kishore and Beatrice Nal
Brunel University London, UK
Posters-Accepted Abstracts: J Infect Dis Ther
Abstract
Soluble molecules of the innate immune system play a major role in the initial defences against infection by pathogens. Properdin is a multimeric soluble protein released by activated neutrophils. It is critical in the stabilisation of the complement alternative pathway C3 convertases. Properdin is able to bind to certain pathogen surfaces, stimulating convertase formation and opsonisation. Our objective was to screen a range of purified human soluble factors involved in innate immunity and complement regulation for their capacity to inhibit haemagglutination of guinea pig red blood cells by human influenza A viruses in a complement-independent manner. Human soluble factors were purified from human serum by affinity chromatography. Alternatively, recombinant proteins were expressed in E.coli. Purified proteins were dialised and purity, molecular weight and appropriate folding were verified. We found that purified properdin inhibits haemagglutination of all influenza A virus strains tested (A/Hong Kong/99 and A/Udorn/72 H3N2 viruses and A/WSN/33, A/Hong Kong/98 and pandemic A/UK/09 H1N1 viruses). Inhibition was more potent on H1N1 viruses including pH1N1. We found that properdin interacts with native viruses and purified haemagglutinin and neuraminidase envelope proteins. Quantitative real-time-PCR studies on M1 viral gene at early and late times post-infection shown that properdin down regulates infection of A549 pneumocytes by pH1N1 virus. Our findings reveal that human properdin binds influenza A virus and restricts infection of target cells. The observation that properdin interacts with influenza A virus without involving C3b is novel and worth further investigation.Biography
Lubna Kouser graduated with a Bachelor’s degree in Biomedical Science from Brunel University in September 2011. Currently, she is a final year PhD student of Dr. Kishore, in Brunel University, mainly focusing on the interaction of human properdin with Mycobacteria and influenza A virus. She has acquired various techniques such as gene cloning, PCR, qPCR, cDNA synthesis, recombinant protein expression in E. coli (globular heads A, B, C, TSRs, SPD, SPA) and baculovirus proteins, immunoassay, Western blots, purification of serum complement proteins: C1q, factor H, properdin, familiar with CL3 facilities and standard operating procedures, microscopy, purification and titration of Influenza A Virus.
Email: Lobna.Kouser@brunel.ac.uk

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi