Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
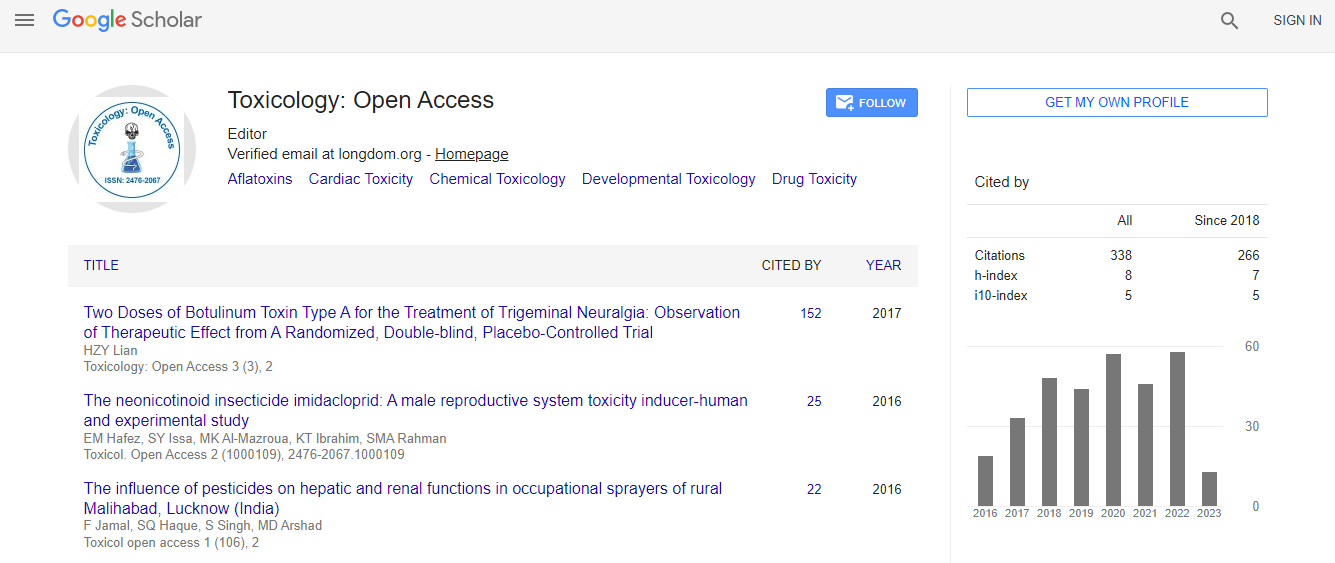
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Preclinical development of biopharmaceuticals (r-DNA Products)
8th World Congress on Toxicology and Pharmacology
Mukul P Pore
Intox Pvt. Ltd., India
Keynote: Toxicol Open Access
Abstract
Biopharmaceuticals are an important component of pharma industry & have grown exponentially in the last decade. The growth is driven by factors such as growing pressure for affordable product development, advances in biochemical and molecular biology instrumentation, growing demand for biosimilar drugs to address ever growing chronic diseases, increasing number of off-patented therapeutics and monoclonals. Great successes were achieved and multiple life-altering therapies were developed for indications like cancer, rare genetic diseases, and immune disorders. Significant guidance has been released by regulatory agencies to help the rational and scientifically based development of these complex products. The goal of preclinical development of biosimilar is to demonstrate that, the drug is √ʬ?¬?highly similar√ʬ?¬? to the reference biologic product in terms of √ʬ?¬?Safety, Efficacy and Quality√ʬ?¬?. Monitoring biopharmaceutical drug safety deserves special attention. In this presentation, challenges in the preclinical development of biologicals (particularly √ʬ?¬?Biosimilars√ʬ?¬?) will discussed in short. Key features of biotechnologyderived molecules (Biosimilars including Vaccines), how they compare to traditional chemical drugs, and the impact these features on preclinical safety testing during their development will also be discussed.Biography
Mukul P Pore is one of the founders and is the Lifetime Director of INTOX Pvt. Ltd. which is a well-known GLP certified contract research organization. He is a Diplomate of the American Board of Toxicology (DABT), European Registered Toxicologist (ERT) and Fellow of Indian Society of Toxicology (FST). He has designed and conducted number of toxicology studies for diverse kind of products - pharmaceuticals, agrochemicals, biotechnology products, specialty chemicals, vaccines, medical devices, industrial chemicals etc., during his experience of over 28 years in regulatory/descriptive toxicology. Since 1996, he has played an important role in establishing and bringing INTOX to international standard and repute. He is an Ad Hoc specialist for AAALAC International, USA (2010-2013; 2013-2016; 2016- 2019). He is member of many professional bodies/societies including Indian Society of Toxicology (STOX), Chinese Society of Toxicology, Japanese Society of Toxicology (JST), UK Registry of Toxicology and Laboratory Animal Scientists Association of India. He was nominated on ‘REACH Expert Committee” as “Expert in the field of Environment, Health and Safety” by Ministry of Chemicals & Fertilizers, Govt. of India (2015). He was nominated as Advisor of Editorial Board of “Toxicology International” journal in 2009.
Email: mukulpore3@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi