Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
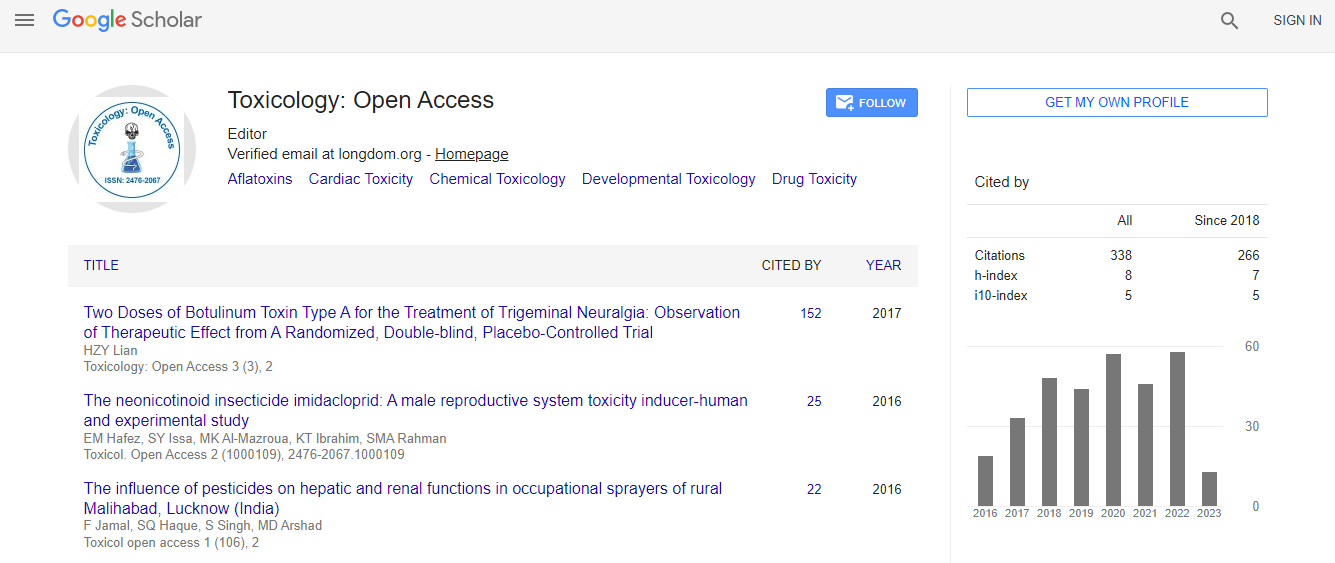
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Reconstructed skin models and methods for hazard and risk assessment of chemicals and cosmetics
8th World Congress on Toxicology and Pharmacology
Christian Pellevoisin
Episkin Academy, France
Keynote: Toxicol Open Access
Abstract
In 2003, the 7th Amendment to the Cosmetics Directive introduced in Europe the regulatory framework for the phasing out of animal testing for cosmetics purposes. Since 2013, this testing and marketing ban fully entered in force and is now part of the European Cosmetic Regulation. Following this European regulation, we observe outside Europe a strong trend for a progressive shift to non-animal methods for safety of ingredients and cosmetics products. Mechanistic approaches to replace the animal are based on in silico, in chemico and in vitro assays that can inform on one or more key events of adverse outcome pathways (AOP). To be as predictive as possible of human being, such individual in vitro test systems rely more and more on cells of human origin with a 3D organization which better mimic the vivo situation. To this point of view, Reconstructed Human Epidermis (RHE) presents several advantages that make it an alternative method of choice for evaluating some safety endpoints. To date, several alternative methods in toxicology have been developed based upon in vitro skin: Skin penetration, skin corrosion/irritation, phototoxicity and genotoxicity. However, an in vitro alternative method must be validated before being recognized by the concerned regulatory bodies. Today, two alternative methods based on in vitro skin models have been validated as full replacement methods to animal, the OECD-TG 431 for in vitro skin corrosion and the OECD-TG 439 for in vitro skin irritation of chemicals. Moreover, two other methods based on human reconstructed epidermis and full thickness models have been submitted for validation in the field of sensitization and genotoxicity.Biography
Christian Pellevoisin, after a PhD in Neuroscience at the French National Institute of Health and Medical Research, had a temporary teaching position at the University of Tours, France. He joined L’Oréal in 2000 at the Life Science Research Center where he introduces computer tools for in vitro toxicology. He was In-charge (2004) of scientific communications in the Field of Alternative Methods and Tissue Engineering. In 2011, he joined EPISKIN, a subsidiary of L’Oréal, dedicated to development and production of reconstructed human epithelia. He is In-charge of EPISKIN Academy, a transversal program to support the use of 3D models for efficiency and safety assessment and to relay EPISKIN commitments to 3Rs by training scientists, students and future stakeholders to the scientific and regulatory challenges of alternative to animal testing. He wrote several scientific publications and is Member of ISO technical committee 194 for biological and clinical evaluation of medical devices.
Email: cpellevoisin@episkin.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi