Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
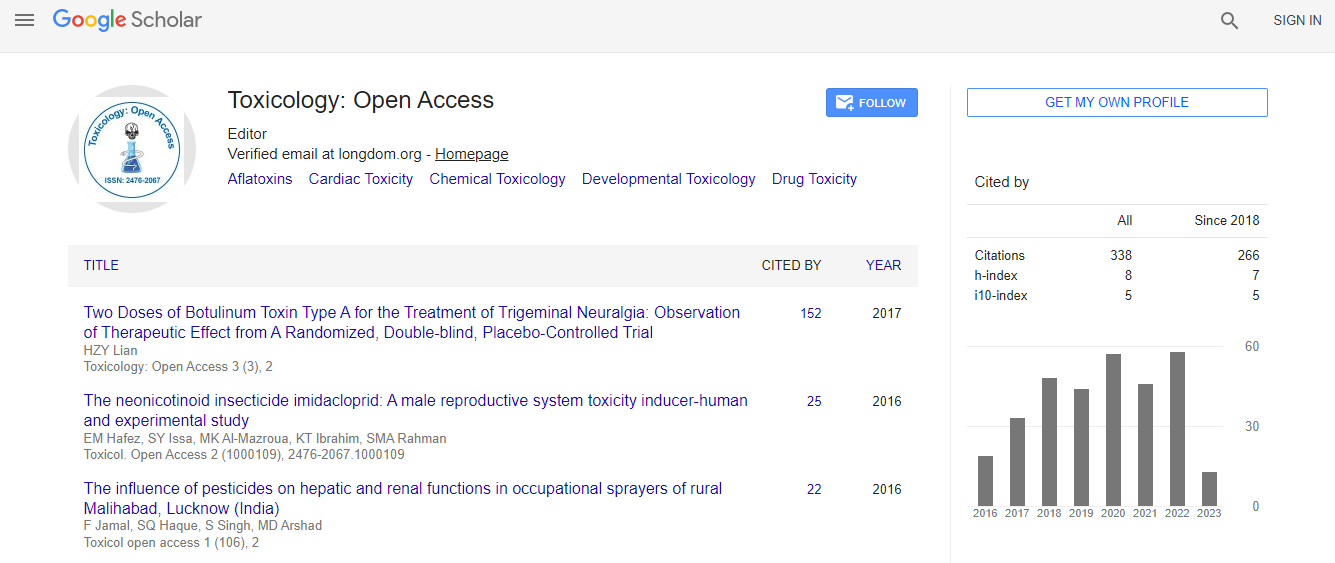
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Snake venom peptides: Potential therapeutic agents in thrombotic diseases
14th World Congress on Toxicology and Pharmacology
Fabio de Oliveira
Federal University of Uberlandia, Brazil
ScientificTracks Abstracts: Toxicol Open Access
Abstract
The snake venoms are constituted of a true biochemical arsenal, consisting of several proteins and peptides with activities that have aroused the curiosity of researchers for centuries, in an attempt to understand its systemic action in order to get pharmacological applications. A number of snake venom proteins that interfere on platelet aggregation have been isolated from these venoms. However, there are no reports in the literature of small peptides interfering in aggregation. In the present work, we identify and characterize, for the first time, a heptapeptide (BaltPAi: platelet aggregation inhibitor from B. alternatus snake venom) and a decapeptide (BmooPAF: platelet-activating factor from B. moojeni snake venom), which potentially inhibits and induces the platelet aggregation, respectively. BmooPAi shows a rather specific inhibitory effect on collageninduced platelet aggregation in human platelet-rich plasma, whereas it has little or no effect on platelet aggregation induced by adenosine diphosphate. The results presented here suggest that the BaltPAi consists of an amino acid sequence present in the C-terminal region of snake venom phospholipase A2 enzymes. This sequence would be responsible for the inhibition of platelet aggregation as well as for the cytotoxicity effects of tumor cells caused by these enzymes. Assays with monoclonal antibodies (anti-integrin ├?┬▒2b and anti-GP1BA) show a significant inhibitory effect on BmooPAF-induced platelet aggregation. On the other hand, anti-GPVI antibody shows no effect on platelet function. These findings, associated with molecular docking, indicate that BmooPAF induces platelet aggregation via binding to the GPIb├?┬▒ platelet receptor leading to ├?┬▒IIb├?┬▓3 integrin activation. These toxins could be of medical interest as tools for the development of novel therapeutic agents for the prevention and treatment of thrombotic disorders. Recent Publications 1. De Queiroz, F de Oliveira, et al. (2017) The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon; 133: 33-47. 2. F de Oliveira, et al. (2016) Biochemical and functional characterization of BmooSP: A new serine protease from Bothrops moojeni snake venom. Toxicon; 111(130): 138.Biography
Fabio de Oliveira has completed his PhD in 2001 from National University of Brasília in Brazil. He was the Director of Innovation and Transfer of Technology of the Federal University of Uberlandia. Currently he is the Professor of the postgraduate program in Genetic and Biochemistry and in Cellular and Structural Biology of the same university. He has experience in the area of biochemistry, biophysics and biotechnology with emphasis in isolation and characterization of pharmacologically active principles present in venom of Brazilian snakes.
Email:fabio@ufu.br

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi