Research Article Open Access
Blood and Tissue Leukocyte Apoptosis in Trypanosoma brucei Infected Rats
Anise N. Happi1,* Danny A. Milner Jr.2 and Richard E. Antia1
1Department of Veterinary Pathology, Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria
2Department of Pathology, Brigham and Women’s Hospital, Boston, MA 02115, USA
- *Corresponding Author:
- Anise N. Happi
Department of Veterinary Pathology
Faculty of Veterinary Medicine
University of Ibadan, Ibadan, Nigeria
E-mail: anisehappi@yahoo.com
Received Date: 29 January 2012 Revised Date: Revised 11 April 2012 Accepted Date: 16 April 2012
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Severity of disease in human African trypanosomiasis may be linked, as in other human protozoan infections, to apoptosis of host inflammatory cells during infection. The present study investigates the involvement of leukocyte apoptosis in Trypanosoma brucei infection of rodents. Twenty-seven Wistar rats were infected with 1 × 103 T. brucei and leukocyte apoptosis was evaluated by several methods. Apoptotic cell count was performed in blood, spleen, thymus, lymph node and liver during infections, using Light Microscopy (LM), agarose gel electrophoresis and Transmission Electron Microscopy (TEM). Blood and tissues leukocyte apoptosis in infected animals were confirmed by LM, TEM, and agarose gel electrophoresis, which showed low molecular weight DNA fragments. Infected rats sacrificed after day 8 PI showed significant increase in apoptotic cells in both blood (p<0.001) and spleen (p < 0.05). Peak blood leukocyte apoptosis corresponded with peak parasitemia and the lowest total leukocyte count in all infected rats. Our data provide the first documentation of increased levels of blood leukocyte apoptosis during T. brucei infection. Apoptosis of blood cells during trypanosome infection may represent a putative mechanism for the severity of leukopenia and disease.
Keywords
Trypanosoma brucei; trypanosomiasis; leukocyte apoptosis; leukopenia
Introduction
African Trypanosomiasis is a major zoonotic disease that detrimentally affects both humans and animals. Fifty to seventy thousand people are estimated to be afflicted by human African trypanosomiasis (HAT) [12]. The disease in humans is universally fatal without treatment. In some areas, the mortality related to HAT is estimated to be in excess of 1000 cases per year [5]. The morbidity and mortality associated with African trypanosomiasis and the severe productivity losses in livestock have a major economic impact in sub- Saharan Africa including the exclusion of use of land due to Glossina fly habitats.
Previous studies have shown that during trypanosomal infection of both susceptible and tolerant animals, there are parasitemia, anaemia, pancytopenia, and phagocytosis of blood cells of all linages in the bone marrow, spleen, liver and lymph nodes [2,3,25]; with resultant increase in hematopoiesis. However, the mechanism is unclear. More than a decade ago, the pioneering work of Moore and Matlashewski [24] first indicated that protozoan parasites (e.g. Leishmania donovani) manipulate apoptotic cell death of their host cells. Since then, the number of parasites which are known to modulate apoptosis within their hosts has continuously expanded and currently includes a variety of protozoans of major medical impact on human health and livestock production [30].
Apoptosis of host cells has been shown to contribute to the pathology of several protozoan infections such as Toxoplasma gondii, Plasmodium spp., Leishmania major, L. donovani, Trypanosoma cruzi, and Cryptosporidium parvum [8,9,20,22,37]. Recently, Silva et al. [31] showed increase in lymphocyte apoptosis in T. cruzi infection in mice. Other investigations have also demonstrated trypanosome-induced apoptosis of the endothelial cell of the brain capillaries in T. brucei infected mice [35]. However, there is little or no knowledge of leukocyte apoptosis in African trypanosomiasis. We hypothesized that T. brucei infection would induce apoptosis of blood and tissue leukocytes and contribute to leukopenia via wide spread phagocytosis in tissues. Findings from our study provided the first demonstration of blood leukocytes apoptosis in T. brucei infected rats using Light and Transmission Electron Microscopy (TEM) as well DNA fragmentation on Gel electrophoresis. The implications of apoptosis of blood and tissue leukocytes during T. brucei infections are discussed.
Materials and methods
Animals, parasites, infection and parasitemia. A total of twenty-seven (27) adult male Wistar rats weighing between 260 g and 280 g were obtained from the Central Animal house, University of Ibadan, and acclimatized for one week prior to experiments. They were checked for trypanosomes and other hemoparasites. T. brucei parasites (Federe strain) used for infections were obtained from the Nigerian Institute for Trypanosomiasis Research (NITR, Vom, Plateau State, Nigeria). This experiment was repeated three times. During the first experiment, 7 infected rats and 2 non-infected controls were used and in the subsequent two experiments, a group of 6 infected and 3 non-infected control rats were used. Infections in rats (19 out of 27) were performed by intraperitoneal injection of 1×103 trypanosomes diluted in 0.2mL of normal saline. Non-infected controls (8 out of 27 rats) were injected with 0.2mL of normal saline only. Whole blood from the tail was used to calculate parasitemia daily in all the infected rats using wet mount preparations and evaluating 20 fields at ×400 light microscopy as described previously [3]. Infected rats were sacrificed at peak parasitemia (108.1–108.7 parasites/mL). Animals were later pooled into 3 groups based on the days of sacrifice as follows: day 8 PI (8 infected rats and 3 non-infected controls); day 10 PI (7 infected rats and 3 non-infected controls), and day 14 PI (4 infected rats and 2 non-infected controls).
Blood and tissues samples collection. Prior to sacrifice, 2mL of whole blood were collected from each rat and transferred to K2 EDTA tubes. From these 2 mL, one mL of the blood was used for hematological analysis and light microscopic evaluation of apoptotic leukocyte, while the other one mL was stored at −20 °C for DNA fragmentation analysis. Animals were subsequently sacrificed by ether asphyxiation. Tissue fragments from spleen, mesenteric lymph node, thymus and liver were collected, transferred into cryotubes and stored at −20 °C for DNA fragmentation analysis. Small pieces (< 5mm in greatest dimension) of tissues from spleen and liver were also harvested and kept in 2.5% gluteraldehyde for electron microscopy examinations. Touch impression smears were also made immediately after sacrifice from the above named tissues for cytological evaluation of apoptotic morphological changes of cells.
Hematological analysis and LM evaluation of apoptotic cell from blood smears. Baseline hematological parameters were determined before infections. Complete blood cells count and packed cell volume (PCV) were determined using capillary filled blood from the rats’ tail [16]. Thin blood smears were prepared immediately after bleeding and stained using Diff Quik stain (Dade Behring Inc., Newark, DE 19714, USA). The stained blood films were used to assess erythrocyte morphology, white blood cells population by differential count and light microscopic evaluation of apoptotic cells. Anaemia was evaluated with the microhematocrit method. Total red blood cell (RBC) and white blood cell (WBC) counts were determined using the Naubawer hemocytometer. Blood apoptotic cell count was determined by counting apoptotic cells per 100 nucleated blood cells.
Cytology preparation and examination. Diff Quik® stained touch impression smears from spleen, liver, mesenteric lymph node and the thymus of non-infected control and T. brucei infected rats were examined using a light microscope at 1000× magnification with oil immersion. The number of apoptotic cells against non-apoptotic cells was evaluated per 500 organ cells and the proportion of apoptotic cell in each group compared with the non-infected control. Images were recorded with a Canon Power shot SD750 digital camera (7.1 Mega Pixel, Canon Inc., Japan).
Electron microscopy preparation and examination. Liver and spleen tissues from 3 non-infected controls and 3 T. brucei infected randomly selected rats were processed for transmission electron microscopic investigation of apoptotic cells, using a standard procedure, as described by Dawes [10]. Three sections from each tissue were examined and the apoptotic cells counted in 14–26 high power fields in each section. The average number of apoptotic cells in the 3 sections was analyzed and comparison made between infected animals and non-infected controls. Images were recorded with an AMT digital camera.
DNA purification from blood and solid tissue cells. DNA extraction and purification from blood and tissues obtained from each animal was performed using the QIAamp DNA Mini kit and DNA blood mini kit (QIAGEN Inc. 27220 Turnberry, Lane, Valencia, USA) as described by the manufacturer’s protocol.
Gel electrophoresis. The level of DNA fragmentation in blood and tissues was evaluated by migrating sixty microliters (60 μL) of DNA obtained from each blood and tissues in 1% agarose gel. One hundred base pair (100 bp) DNA ladder was used as reference. The gel was visualized under the UV transilluminator light at 325 nm wavelength.
Statistical analysis. Data analyses were performed using the GraphPad Prism® version 4.0 for Windows software (GraphPad Software, San Diego, CA, USA, www.graphpad.com). Student T test was used to compare the differences in apoptotic cell counts in blood and tissue cells between infected and non-infected control animals. One way analysis of variance (ANOVA) was also used to compare the mean apoptotic cell count in the blood and the selected tissues between the three groups of infected and the non-infected rats. Mean values were presented as mean ± standard deviation. p values ≤ 0.05 were considered significant.
Results
Apoptosis in peripheral blood and tissues was detected during the Parasitemia, hematology and blood cell apoptotic changes in T. brucei infected rats. Infection lasted for 7–14 days and the average peak parasitemia was 108.2 trypanosomes/mL. Peak parasitemia occurred between day 6 and 12 postinfection and varied between 108.1 and 108.7/mL. Anaemia was observed at peak parasitemia together with a moderate leukopenia and thrombocytopenia. The leukopenia was due to lymphopenia and neutropenia (Table 1). Significant monocytosis was also observed in T. brucei infected group day 14 (p = 0.009) compared to non-infected control and day 8 groups.acute T. brucei infection in rats.
| Infected | |||
|---|---|---|---|
| Controls(n = 8) | Day 8 PI(n = 11) | Day 13 PI | |
| (n = 4) | |||
| PCV(%) | 49.91 ± 4.89 | 33.27 ± 7.157 | 22.92 ± 3.520 |
| Total RBC (×106 /µL) | 8.15 ± 0.84 | 5.804 ± 1.335 | |
| Total WBC (×103 /µL) | 10.775 ± 1.554 | 6.314 ± 1.381 | 6.450 ± 1.598 |
| Lymphocytes %, | 64.8 ± 13.48 | 71.83 ± 6.43 | 61.67 ± 5.859 |
| absol | 6604 ± 1587 | 4400 ± 1002 | 3920 ± 605.2 |
| Seg Neut %, | 32.8 ± 15.21 | 24 ± 8.27 | 22.33 ± 1.528 |
| absol | 3481 ± 1906 | 1412 ± 375.9 | 1431 ± 294.5 |
| Monocytes %, | 1.8 ± 1.30 | 2.16 ± 1.17 | 13.67 ± 5.508 |
| absol | 183.2 ± 127.4 | 136 ± 83.12 | 937.3 ± 617.3∗ |
| Eosinophils %, | 1 ± 1 | 0.17 ± 0.41 | 0 ± 0 |
| absol | 91.2 ± 86.93 | 12.5 ± 30.62 | 0.0 ± 0.0 |
| Basophils %, | 0.8 ± 1.09 | 1.67 ± 1.86 | 1.667 ± 1.528 |
| absol | 68.4 ± 94.11 | 137.6 ± 128.3 | 107.0 ± 92.77 |
*Statistically significant change from non-infected control.
Seg Neut: segmented neutrophils.
Absol: absolute count.
All values shown are mean ± standard deviation.
Table 1: Haematology of T. brucei infected and noninfected control rats.
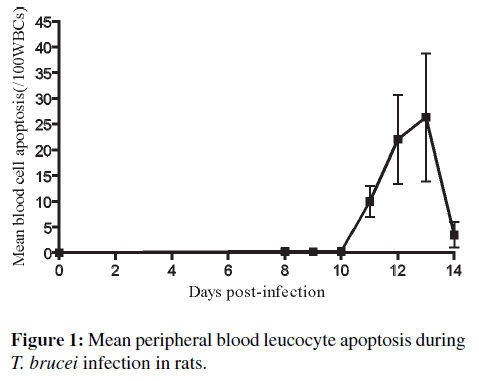
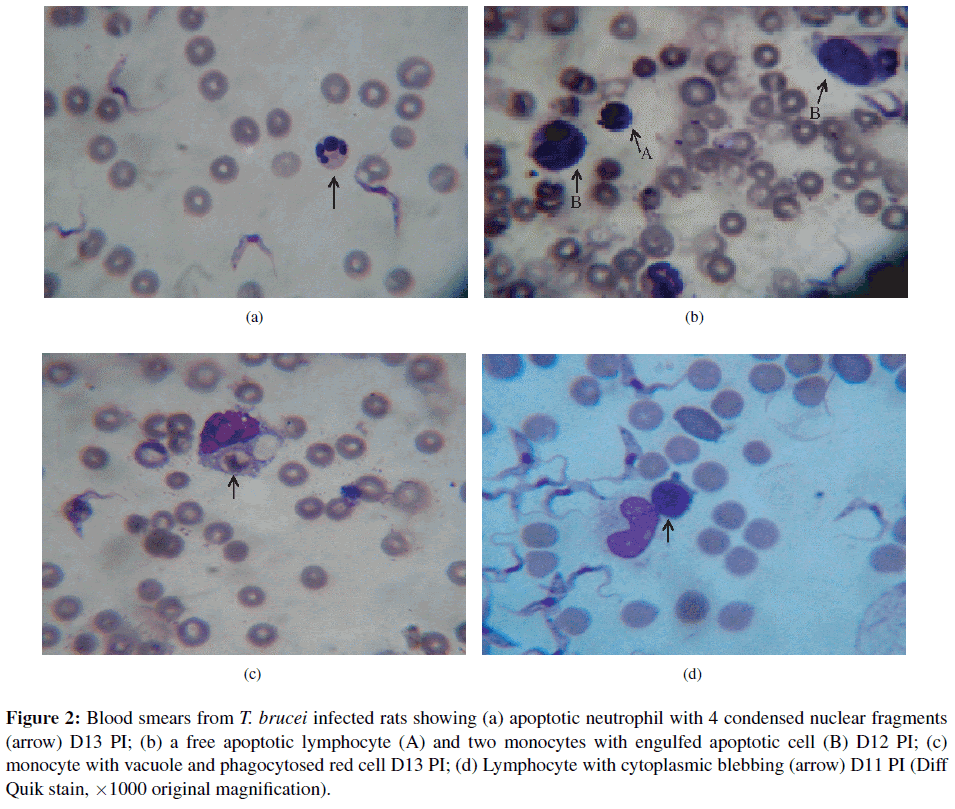
Blood cell apoptotic detection using LM examination of blood smears in T. brucei infected rats. Blood cell apoptosis was first noticed in the blood smear of one out of the 19 infected rats at day 8 PI. There was gradual increase in the pattern of apoptotic cell death in the four rats of the group day 14 PI. The percentage of apoptotic cells in blood increased to a peak of 26.3/100 WBC at day 13 PI and then dropped towards an average of 3.5/100 WBC on day 14 PI (Figure 1). Blood cell apoptosis increased significantly (p < 0.001) in T. brucei infected rats between days 12–13 PI compared with non-infected controls. Although, it was difficult to tell within which of the cell lines apoptosis was occurring, they were recognized to be mostly lymphocytes and a few neutrophils. Blood apoptotic cells generally appeared smaller with condensed nuclear chromatin; others showed nuclear fragmentation (Figure 2(a)) and some pleomorphic, elongated nuclei with clumped nuclear chromatin. Also, the apoptotic cell’s cytoplasm was uniformly eosinophilic. In addition, about 10% of monocytes were vacuolated. Two out of 4 infected rats that survived up to day 14 PI also had monocytes that contained engulfed shrunken cells showing pyknotic nuclei (apoptotic cells) (Figure 2(b)). In three infected animals, monocytes with phagocytosed red blood cell were also seen (Figure 2(c)). A few lymphocytes (2–3% of normal lymphocytes) from four infected rats with a very high parasitemia revealed shrinkage of the cytoplasm with condensed nuclear chromatin and cytoplasmic blebbing (Figure 2(d)) between days 12–14 PI compared to none in non-infected controls. No apoptotic cells were observed in all blood smears made from the 8 non-infected control rats. No morphological change during the infection was detected in other cells with light microscopy. Other light microscopy findings revealed a few bi-nucleated lymphocytes; lymphocytes with azurophilic granules; and reactive lymphocytes from blood smear examination of T. brucei infected rats.
Figure 2: Blood smears from T. brucei infected rats showing (a) apoptotic neutrophil with 4 condensed nuclear fragments (arrow) D13 PI; (b) a free apoptotic lymphocyte (A) and two monocytes with engulfed apoptotic cell (B) D12 PI; (c) monocyte with vacuole and phagocytosed red cell D13 PI; (d) Lymphocyte with cytoplasmic blebbing (arrow) D11 PI (Diff Quik stain, ×1000 original magnification).
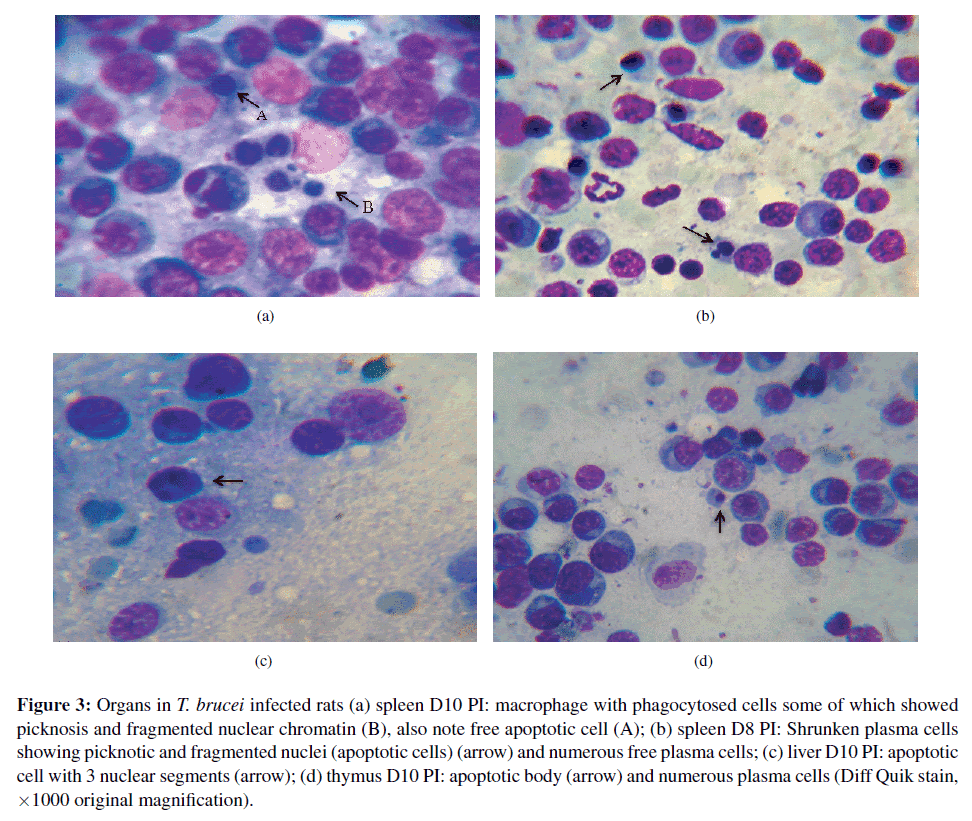
Evaluation of apoptotic changes in cytologic preparations of organs in T. brucei infected rat. Cytological examination of the spleen, liver, thymus and mesenteric lymph node revealed that a total of 12 out of the 19 infected rats showed numerous plasma cells (average of 2–6 per high power field). As expected, an increase in macrophages and a few mast cells, M¨ott cells as well as moderate neutrophils and active macrophages were observed in all the organs examined in infected rats. Parasites were also found in those organs of infected rats. Macrophages contained phagocytosed cells (i.e. red cells, and in some cases lymphocytes, plasma cells, and cell debris). Free apoptotic cells were also observed of varying sizes with loss of cell cytoplasm and shrunken nuclear morphology. The apoptotic changes were deeply basophilc cytoplasm (in the case of lymphocytes), perinuclear and condensed chromatin, and in some cases nuclear fragmentation. Apoptotic bodies were also found in various organs of infected rats from day Spleen. In the spleen, there were free spleen cells (splenocytes) showing apoptotic changes in about 78% of all infected rats (Figure 3(a)). These splenocyte apoptotic changes increased gradually from the non-infected values (0/500 cells) to a peak (9.3±3.7/500 cells) at day 10 PI and this increase was maintained until day 14 PI (9.5±4.5/500 cells). The proportion of apoptotic splenocyte increased significantly on days 10 (p = 0.007) and 14 (p = 0.0033) in infected groups of rats compared to non-infected controls (Table 2). Spleen smears from infected rats also showed numerous plasma cells and lymphocytes of various sizes and nuclear condensation. In addition, free apoptotic splenocytes (Figure 3(a)A) and plasma cells (Figure 3(b)) were observed. Furthermore, moderate numbers of macrophages with phagocytosed red cells, hemosiderin, apoptotic lymphocytes (Figure 3(a)B), plasma cells and or cell debris were found in the spleen smear of T. brucei infected rats. No apoptotic cells were observed in uninfected controls. 10 PI.
Figure 3: Organs in T. brucei infected rats (a) spleen D10 PI: macrophage with phagocytosed cells some of which showed picknosis and fragmented nuclear chromatin (B), also note free apoptotic cell (A); (b) spleen D8 PI: Shrunken plasma cells showing picknotic and fragmented nuclei (apoptotic cells) (arrow) and numerous free plasma cells; (c) liver D10 PI: apoptotic cell with 3 nuclear segments (arrow); (d) thymus D10 PI: apoptotic body (arrow) and numerous plasma cells (Diff Quik stain, ×1000 original magnification).
Liver. Forty seven per cent (47%) of livers from all infected rat showed apoptosis of lymphocytes and plasma cells. Some plasma cells as well as lymphocytes showed fragmented nuclei (Figure 3(c)). There was also cell shrinkage, with condensed chromatin and plasma cell apoptotic bodies. The difference in number of apoptotic cells over time did not reach statistical significance (p > 0.05) (Table 2). No apoptotic cells were observed in uninfected controls.
| N | Spleen(/500 cells) | Liver(/500 cells) | Thymus(/500 cells) | Lymph node(/500 cells) | |
|---|---|---|---|---|---|
| Control | 8 | 0 | 0 | 0 | 0.4 ± 0.4 |
| Day 8 PI | 8 | 4 ± 1.9 | 1.3 ± 0.7 | 2.1 ± 1.0 | 2.1 ± 1.1 |
| Day 10 PI | 7 | 9.3 ± 3.7 | 2.1 ± 1.2 | 7.0 ± 4.0 | 3.2 ± 2.4 |
| Day 14 PI | 4 | 9.5 ± 4.5 | 12 ± 10.5 | 3.0 ± 1.7 | 0.8 ± 0.5 |
| Apoptotic cells(% rats) | 78% | 47% | 57% | 47% | |
| p value | 0.0455* | 0.0505 | 0.1788 | 0.4907 |
Mean ± standard error.
*mean apoptotic cell significantly different from non-infected value.
N = number of rat.
Table 2: Cytological evaluation of apoptotic cells in tissues of T. brucei infected rat.
Thymus. The thymus of T. brucei infected rats revealed lymphocytes of different stages of development and a few large bi-nucleated cells (lymphocytes). The thymus also showed macrophages with phagocytosed lymphocytes and parasites. In addition, the thymus of T. brucei infected rats showed a few dividing cells (lymphoid series), M¨ott cells, mast cells, apoptotic thymocytes and apoptotic bodies (Figure 3(d)). In total, 57% of infected rat thymus revealed cellular apoptosis with light microscopy. The increase in apoptotic cell count in T. brucei infected rats was not significant (p > 0.05). No apoptotic cells were observed in uninfected controls.
Mesenteric lymph node. The lymph nodes of T. brucei infected rats showed numerous lymphoglandular bodies and moderate epithelioid macrophages with blue stained cytoplasm and some containing phagocytic cellular debris. Small plasma cells showing fragmented nuclei were present; condensed chromatin and other uncharacterized apoptotic cells were also observed. All together, 47% of lymph nodes from infected rats revealed apoptotic changes. The lymph nodes change in apoptotic cells over time was significant (p < 0.05) (Table 2).
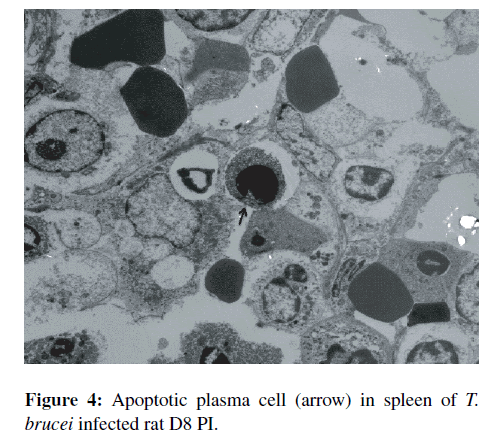
TEM evaluation of apoptotic cells from spleen and liver of T. brucei infected rats. Various stages of apoptosis were detected in sections of the spleen and in the liver. The apoptotic cells appeared singly and were shrunken with some showing fragmented nuclei as well as asymmetric condensation of the chromatin. Apoptotic bodies were often observed in the organs of infected animals. TEM of the spleens sections of T. brucei infected rats revealed reactive macrophages with vesicular nuclei and apoptotic cells. The spleen sections of non-infected control rats showed fewer reactive macrophages, more red pulp and fewer apoptotic cells. There was an average of 2.68 apoptotic cells per high power field (hpf) in infected rats spleen compared to 0.5/hpf in non-infected control rat spleen. Overall, there was a significant increase in splenocyte apoptosis in T. brucei infected rats compared to non-infected control (p < 0.001). The apoptotic cells were mainly lymphocytes with a few plasma cells (Figure 4). TEM sections of the livers from T. brucei infected rats showed more apoptotic cells compared to non-infected controls, although the difference was not statistically significant (p > 0.05).
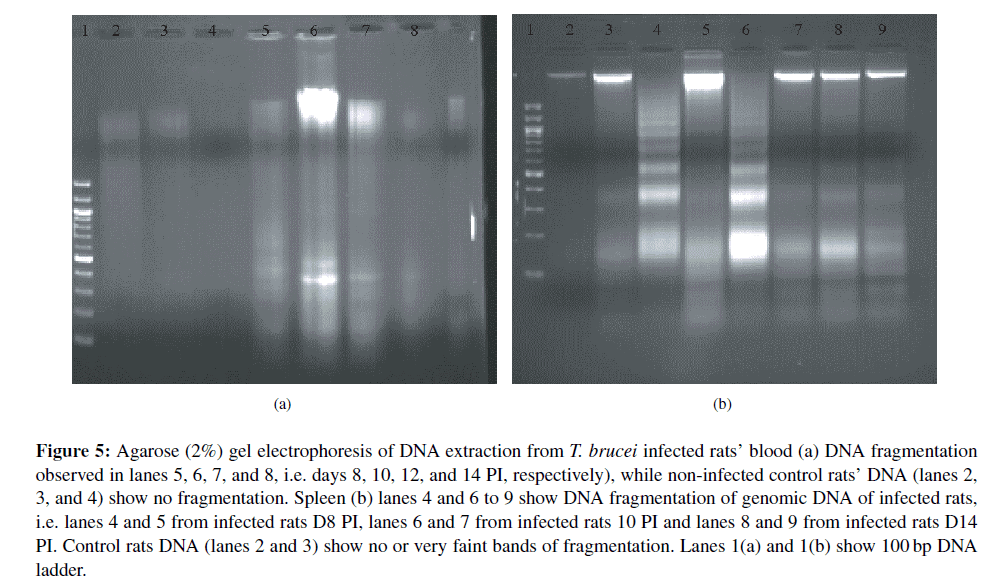
Evaluation of DNA fragmentation from blood and tissue cells of T. brucei infected and non-infected control rats. DNA fragmentation indicative of apoptosis in the blood of infected rats was observed from day 10 PI. Blood cell DNA bands of all the infected rats here examined revealed endonucleosomal fragmentation compared to none in the blood DNA of non-infected control rats (Lanes 2, 3, and 4) (Figure 5(a)). Spleen extracts revealed clear bands of DNA fragmentation in most T. brucei infected rats compared to non-infected controls (lanes 4, 6, and 8; Figure 5(b)). This result demonstrated clearly that there is increase in apoptosis in T. brucei infected rats spleen compared the non-infected control spleen. Liver, thymus and lymph node from both non-infected controls and T. brucei infected animals showed DNA fragmentation with no visual distinction (result not shown).
Figure 5: Agarose (2%) Gel electrophoresis of DNA extraction from T. brucei infected rats’ blood (a) DNA fragmentation observed in lanes 5, 6, 7, and 8, i.e. days 8, 10, 12, and 14 PI, respectively), while non-infected control rats’ DNA (lanes 2, 3, and 4) show no fragmentation. Spleen (b) lanes 4 and 6 to 9 show DNA fragmentation of genomic DNA of infected rats, i.e. lanes 4 and 5 from infected rats D8 PI, lanes 6 and 7 from infected rats 10 PI and lanes 8 and 9 from infected rats D14 PI. Control rats DNA (lanes 2 and 3) show no or very faint bands of fragmentation. Lanes 1(a) and 1(b) show 100 bp DNA ladder.
Relationship between blood cell apoptosis with parasitemia and leukocyte counts in T. brucei infected rats. There was no relationship between leukocyte apoptosis and the level of parasitemia. There was a sustained parasitaemic height during the course of infection in T. brucei infected rats, while blood cell apoptosis in infected animals began at day 8 PI and reached their peak (26.3/100 WBC) between days 12 and 13 PI. T. brucei infected rats which survived longer showed more apoptosis of blood cells and apoptosis of spleen cells. During that period, significant increase in monocyte count (p = 0.0006) was observed with a peak at day 13 PI. The total WBC, lymphocytes and neutrophils count decreased significantly when the percentage blood cell apoptosis began to increase (Figure 6). However, monocyte count increased with the percentage blood cell apoptosis (Figure 6).
Comparison of organ cell apoptosis and duration of infection and blood cell apoptosis in T. brucei infected rats. There was a gradual increase in apoptotic cells in all the organs examined till day 10 PI. However, from D10 apoptotic cell count dropped in the thymus and mesenteric lymph node, while that of spleen cells was maintained until the end of the study at day 14 PI. Apoptosis of lymphoid cells in the liver continued to increase until D14 but not significantly as the standard deviation between animals abrogated the increase. Blood cell apoptosis on the other hand increased from D8 until day 13 PI.
Discussion
This study demonstrated trypanosome-induced peripheral blood and tissue cell apoptosis in rats, providing a potential insight in the pathogenesis of T. brucei infections in animals.
Trypanosome-induced peripheral blood cell apoptosis has never been documented as a feature of trypanosomiasis. During this study white blood cell (lymphocytes and neutrophils) death by apoptosis was detected in T. brucei infected animals by LM examination and confirmed by the presence of ladders of oligonucleosomes on DNA Gel electrophoresis. In most parasitic disease conditions, blood cell apoptosis is very rare to find by light microscopy examination as they are rapidly engulfed by adjacent cells such as monocytes [28]. Peripheral blood leukocyte apoptosis was more readily noticeable in infected rats that survived longer; this may suggest an increase in apoptotic cells or a defect in their clearance. While an increase in apoptotic cells is most probable, it was observed that during that same period there was an increase in the main blood phagocytic cells (monocytes) and the reduction in the leukocyte count. Blood cell apoptosis has been reported in cancer [32], viral infections [14], bacterial diseases [7], AIDS (acquired immunodeficiency syndrome) [21,41]) and induced by drugs [33, 34]. Apoptotic cell death has also been reported in non-pathological conditions and constitutes part of a mechanism of cell replacement and tissue remodeling leading to maintenance of homeostasis [18].
Furthermore, trypanosome infections produced marked alterations in the morphology and number of erythrocytes in this study. Anemia has been described in T. brucei infection in all susceptible hosts and laboratory animals [23]. In addition, leukopenia, thrombocytopenia, lymphopenia and monocytosis were observed in infected rats. These findings are consistent with previous reports [15,17] and are prominent features of animal trypanosomiasis. Furthermore, vacuolated monocytes, some of which engulfed red cells, were also found in blood of infected animals. These have been reported in tissues (spleen, liver, lymph nodes and bone marrow) of trypanosoma-infected animals where macrophages engulfed red cells with hemaosiderin and other blood cells [2,36].
In this study it was found that lymphopenia was as a result of increase lymphocyte apoptotic death, as a significant (p < 0.001) number of apoptotic lymphocytes were recorded at later stages of T. brucei infected rats.
It has also been reported that infection of mice with T. cruzi led to induction of both T [22] and B cell apoptosis [42] and contribute to proliferation of parasites and lymphopenia. Although the reason for appearance of blood cell apoptosis during T. brucei infection is still not clear, it has been reported in various diseases conditions such as leukaemia [19], HIV [39], and bacterial diseases [7]. Apoptosis of blood cells has been found to be induced in vitro by therapeutic drugs [26].
Monocytopenia was observed at day 8 PI but a significant increase in monocyte count (monocytosis) above the pre-infection value was recorded afterward. This correlated with the significant increase in the number of apoptotic blood cells. The increase in monocyte count may be as a result of the host response to control the infection and in so doing may prime lymphocyte for apoptosis. Moreover, blood cell apoptosis did not correlate with the level of parasitemia, but rather correlated with the duration of infection and monocyte count. Wu et al. [40] have reported that monocytes are required to prime peripheral blood T cells to undergo apoptosis especially upon polyclonal stimulation of T cells, this also suggest the possibility of the similar phenomenon in T. brucei infected host peripheral blood lymphocyte. Although the findings from the present study demonstrate for the first time the correlation between blood cell apoptosis and monocytosis, they may further explain a previous report by Anosa [1], who suggested that monocytosis usually occurs when the workload of the system increases such as in the presence of trypanosomes and damage red cells. The need for phagocytosis of cells programmed to die in blood stream of T. brucei infected hosts might also stimulate the production of more monocytes in circulation.
Neutropenia was shown in infected animals during this study, suggesting a contribution of neutrophil apoptosis to neutropenia in trypanosomiasis. A previous study [4] suggested that RBC protect against neutrophil apoptosis in circulation by reducing intracellular oxidant stress through catalase and glutathione metabolism. Knowing that anaemia is a consistent finding in trypanosomal infection, the decreased RBC count will then lead to a decrease in amount of antioxidants and consequently the decrease in protective effect against apoptosis of circulating neutrophils. Neutropenia has been reported during trypanosomiasis [2,38]. The reduction in neutrophil count associated with neutrophilic apoptotic death observed in this study also suggests that reduction in number or absence of neutrophils in tissues during acute inflammatory response of trypanosomiasis could be associated with suppressive effect of anemia. The decrease in protecting oxidant due to anaemia may induce apoptosis of circulating neutrophils.
LM, DNA fragmentation assay and TEM results showed increases in splenocyte apoptosis in T. brucei infected rats and this agrees with the study of Radwanska et al. [27] that T. brucei infected mouse showed spleen cell (B cell) apoptosis during the early onset of parasitemia with resultant loss of protective anti-parasite antibody responses. More specifically, it has recently been reported that in the spleen, final B cell maturation is abrogated by T. brucei induced apoptosis of transitional B cells of both the T1 and T2 populations [6].
The correlation between the increase in macrophages and the significant increase in apoptotic spleen cells in T. brucei infected animals compared to non-infected controls observed might suggest the increase need for phagocytosis of parasite and damaged cells such as numerous apoptotic splenocytes. The spleen is the major site for phagocytic removal of foreign or unwanted materials in general. An important consequence of the apoptotic process is cell surface alterations that lead to rapid recognition and phagocytosis of apoptotic cells by macrophages [29]. In addition, the peak apoptosis coincides with the peak parasitemia; furthermore, macrophages with numerous phagocytosed apoptotic lymphocyte and plasma cells were observed during late stage of infection. This might imply that during the early stage of infection the apoptosis induced cell are quickly phagocytosed by the still efficient and many adjacent or tissues phagocytic cells. During the later stage of infection, as the immune system is weakened by apoptotic death of splenocyte, the phagocytic cells are overwhelmed; thus), more free and phagocytosed apoptotic cells are seen. These in turn may facilitate parasite proliferation with subsequent rise in parasitemia.
The cytological and TEM examinations of the liver revealed an increase in lymphocyte apoptosis during infection, but this was not statistically significant. This result also confirmed by the agarose Gel electrophoresis of DNA fragmentation assay showed no difference between the 2 groups. The reasons behind the non-significant increase in liver lymphocyte apoptosis remain unclear. The targeted cells for apoptosis (lymphocytes and to a lesser extend neutrophils) are not the main resident cells of the liver as in the spleen and thymus. The increase in apoptosis of thymocytes in infected animals could contribute to lymphopenia through it direct effect on T cells. It has been shown that apoptotic cells appear rarely in vivo, even in tissues such as the thymus in which extensive apoptosis is a normal feature during regression [13]. Increase in thymocyte apoptosis, however small, therefore could contribute to immune suppression of the host. This non-significant difference observed could also be due to the rapid phagocytosis of apoptotic cells in vivo as only the free apoptotic cells were counted. Furthermore, the nonsignificant increase in thymocyte apoptosis could also be because the main target cells are B cells, as Radwanska et al. [27] reported that T. brucei infection induces polyclonal B cell activation; B cell clonal exhaustion, sustained depletion of mature splenic Marginal Zone B and Follicular B cells; and destruction of the B-cell memory compartment.
Smaller plasma cells showed fragmented nuclei; condensed chromatin and lymphocytes apoptotic cells were also observed. Although the number of cells undergoing apoptosis was modest in the mesenteric lymph node (MLN) of T. brucei infected rats, it was more than in non-infected control rats. Some authors have reported apoptosis of T and B cells from MLN in the course of T. cruzi infection [11]. It has been reported that during T. brucei infection of mice there is rapid loss of B cells (through apoptosis) in the spleen and consequently loss of B cell responsiveness which prevent the induction of protective memory responses [27].
Although the results herein discussed are from investigation of acute uncontrolled infection in laboratory model, as these scenarios may not be exactly similar with that of the natural host, particularly as the infection in the natural host runs a chronic course and is associated with a much lower parasitemic height. The strength of this study however, is that the apoptotic level correlated with leukopenia throughout the six day interval during which the parasitemic level was relatively steady, so apoptosis is less likely to the result of model-specific stress in preterminal animals. In addition, even if apoptosis is higher in this model than in natural infection, it may still be a significant mechanism, since cells primed to undergo programmed cell death are quickly engulfed by adjacent cells or macrophages and are therefore rendered invisible due to efficient phagocytosis. This may have considerable impact on the level of apoptotic cell detection. However, additional research will be needed to investigate this phenomenon in the natural host infected with T. brucei.
In conclusion, the results presented in this study show that there is an increase in blood leukocyte and spleen cell apoptosis in T. brucei infected rats. A major breakthrough of this study is the first documentation of blood leukocyte apoptosis during T. brucei infection. Therefore, apoptosis may have a role in limiting the host response, which may results in increased parasite growth. The identification of mechanisms that mediate the induction of apoptotic cell death during trypanosomiasis might suggest novel disease intervention strategies.
Acknowledgments
D. A. Milner Jr. was supported by Exxon Mobil Foundation through the Harvard School of Public Health. We thank Mr. Olatunde Matthew and Prof. A. Sowunmi of the Malaria Research Laboratories, College of Medicine, University of Ibadan for technical assistance. We also thank Prof. C. T. Happi for valuable contribution and suggestions in the manuscript.
References
- V. O. Anosa, Studies on the parasitaemia, plasma volumes, leucocyte and bone marrow cell counts, and the moribund state in Trypanosoma brucei infection of splenectomised and intact mice,Zentralbl Veterinarmed B, 27 (1980), 169–180.
- V. O. Anosa and J. J. Kaneko, Pathogenesis of Trypanosoma brucei infection in deer mice (Peromyscus maniculatus): hemato-logic, erythrocyte biochemical, and iron metabolic aspects, Am JVet Res, 44 (1983), 639–644.
- V. O. Anosa, L. L. Logan-Henfrey, and M. K. Shaw, A light and electron microscopic study of changes in blood and bone marrow in acute hemorrhagic Trypanosoma vivax infection in calves, VetPathol, 29 (1992), 33–45.
- K. Aoshiba, Y. Nakajima, S. Yasui, J. Tamaoki, and A. Nagai, Red blood cells inhibit apoptosis of human neutrophils, Blood,93 (1999), 4006–4010.
- M. P. Barrett, D. W. Boykin, R. Brun, and R. R. Tidwell, Human African trypanosomiasis: pharmacological re-engagement with a neglected disease, Br J Pharmacol, 152 (2007), 1155–1171.
- V. Bockstal, P. Guirnalda, G. Caljon, R. Goenka, J. C. Telfer, Frenkel, et al., T. brucei infection reduces B lymphopoiesis in bone marrow and truncates compensatory splenic lymphopoiesis through transitional B-cell apoptosis, PLoS Pathog, 7 (2011),e1002089.
- J. A. Carrero and E. R. Unanue, Lymphocyte apoptosis as an immune subversion strategy of microbial pathogens, TrendsImmunol, 27 (2006), 497–503.
- X. M. Chen, G. J. Gores, C. V. Paya, and N. F. LaRusso, Cryptosporidium parvum induces apoptosis in biliary epithelia by a Fas/Fas ligand-dependent mechanism, Am J Physiol, 277(1999), G599–G608.
- G. Das, H. Vohra, K. Rao, B. Saha, and G. C. Mishra, Leishmania donovani infection of a susceptible host results in CD4+ T-cell apoptosis and decreased Th1 cytokine production, Scand JImmunol, 49 (1999), 307–310.
- C. J. Dawes, Biological Techniques in Electron Microscopy, Barnes & Noble, New York, 1979.
- J. de Meis, D. A. Mendes-da Cruz, D. A. Farias-de Oliveira, Correaˆ-de Santana, F. Pinto-Mariz, V. Cotta-de Almeida, et al., Atrophy of mesenteric lymph nodes in experimental Chagas’ disease: differential role of Fas/Fas-L and TNFRI/TNF pathways,Microbes Infect, 8 (2006), 221–231.
- FAO (Food and Agricultural Organization), Programme Against African Trypanosomosis (PAAT). http://www.fao.org/ag/againfo/programmes/en/paat/home.html, 2011.
- A. Hague and C. Paraskeva, Apoptosis and disease: a matter of cell fate, Cell Death Differ, 11 (2004), 1366–1372.
- E. Holznagel, R. Hofmann-Lehmann, C. M. Leutenegger, Allenspach, S. Huettner, U. Forster, et al., The role of in vitro-induced lymphocyte apoptosis in feline immunodeficiency virus infection: correlation with different markers of disease progression, J Virol, 72 (1998), 9025–9033.
- T. T. Isoun, The histopathology of experimental disease produced in mice infected with Trypanosoma vivax, Acta Trop, 32 (1975),267–272.
- N. C. Jain, Schlam’s Veterinary Hematology, Lea & Febiger, Philadelphia, PA, 4th ed., 1986.
- F. W. Jennings, P. K. Murray, M. Murray, and G. M. Urquhart, Anaemia in trypanosomiasis: studies in rats and mice infected with Trypanosoma brucei, Res Vet Sci, 16 (1974), 70–76.
- J. F. Kerr, A. H. Wyllie, and A. R. Currie, Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics, Br J Cancer, 26 (1972), 239–257.
- M. Leno, R. M. Simpson, F. S. Bowers, and T. J. Kindt, Humanlymphocyte virus 1 from a leukemogenic cell line mediates in vivo and in vitro lymphocyte apoptosis, J Exp Med, 181 (1995),1575–1580.
- O. Liesenfeld, J. C. Kosek, and Y. Suzuki, Gamma interferon induces Fas-dependent apoptosis of Peyer’s patch T cells in mice following peroral infection with Toxoplasma gondii, InfectImmun, 65 (1997), 4682–4689.
- R. G. Lima, J. Van Weyenbergh, E. M. Saraiva, M. Barral-Netto, B. Galvao˜ Castro, and D. C. Bou-Habib, The replication of human immunodeficiency virus type 1 in macrophages is enhanced after phagocytosis of apoptotic cells, J Infect Dis., 185(2002), 1561–1566.
- M. Lopes, V. da Veiga, A. Santos, M. Fonseca, and G. DosReis, Activation-induced CD4+ T cell death by apoptosis in experi-mental Chagas’ disease, J Immunol, 154 (1995), 744–752.
- G. J. Losos and B. O. Ikede, Review of pathology of diseases in domestic and laboratory animals caused by Trypanosoma congolense, T. vivax, T. brucei, T. rhodesiense and T. gambiense,Vet Pathol, 9 (1972), 1–79.
- K. J. Moore and G. Matlashewski, Intracellular infection by Leishmania donovani inhibits macrophage apoptosis, J Immunol,152 (1994), 2930–2937.
- J. Naessens, Bovine trypanotolerance: A natural ability to prevent severe anaemia and haemophagocytic syndrome?, Int JParasitol, 36 (2006), 521–528.
- K. Nagami, Y. Kawashima, H. Kuno, M. Kemi, and H. Mat-sumoto, In vitro cytotoxicity assay to screen compounds for apoptosis-inducing potential on lymphocytes and neutrophils, JToxicol Sci, 27 (2002), 191–203.
- M. Radwanska, P. Guirnalda, C. De Trez, B. Ryffel, S. Black, and S. Magez, Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abol-ishment of vaccine-induced memory responses, PLoS Pathog, 4(2008), e1000078.
- J. Savill and V. Fadok, Corpse clearance defines the meaning of cell death, Nature, 407 (2000), 784–788.
- J. S. Savill, A. H. Wyllie, J. E. Henson, M. J. Walport, P. M. Henson, and C. Haslett, Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages, J Clin Invest,83 (1989), 865–875.
- F. Schaumburg, D. Hippe, P. Vutova, and C. G. Luder,¨ Pro- and anti-apoptotic activities of protozoan parasites, Parasitology, 132(2006), S69–S85.
- E. Silva, L. Guillermo, F. Ribeiro-Gomes, J. De Meis, M. Nunes, Senra, et al., Caspase inhibition reduces lymphocyte apoptosis and improves host immune responses to Trypanosoma cruzi infection, Eur J Immunol, 37 (2007), 738–746.
- T. A. Simao,˜ M. J. Andrada-Serpa, G. A. Mendonc¸a, D. D. Marques, M. A. Braga, A. L. Santos, et al., Detection and analysis of apoptosis in peripheral blood cells from breast cancer patients, Braz J Med Biol Res, 32 (1999), 403–406.
- E. Solary, L. Dubrez, and B. Eymin, The role of apoptosis in the pathogenesis and treatment of diseases, Eur Respir J, 9 (1996),1293–1305.
- K. Stahnke, S. Fulda, C. Friesen, G. Strauss, and K. M. Debatin, Activation of apoptosis pathways in peripheral blood lymphocytes by in vivo chemotherapy, Blood, 98 (2001), 3066–3073.
- J. K. Stiles, J. Whittaker, B. Y. Sarfo, W. E. Thompson, D. Powell, and V. C. Bond, Trypanosome apoptotic factor mediates apoptosis in human brain vascular endothelial cells,Mol Biochem Parasitol, 133 (2004), 229–240.
- K. Taylor and E. M.-L. Authie,´ Pathogenesis of animal trypanosomiasis, in The Trypanosomiases, I. Mauldin, P. H.Holmes, and M. A. Miles, eds., CABI, Oxfordshire, UK, 2004, 331–354.
- A. Toure-Balde, J. L. Sarthou, G. Aribot, P. Michel, J. F. Trape, Rogier, et al., Plasmodium falciparum induces apoptosis in human mononuclear cells, Infect Immun, 64 (1996), 744–750.
- V. E. Valli, C. M. Forsberg, and J. H. Lumsden, The pathogenesis of Trypanosoma congolense infection in calves. III. Neutropenia and myeloid response, Vet Pathol, 16 (1979), 96–107.
- M. O. Westendorp, R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, et al., Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120, Nature, 375 (1995),497–500.
- M. X. Wu, J. F. Daley, R. A. Rasmussen, and S. F. Schlossman, Monocytes are required to prime peripheral blood T cells to undergo apoptosis, Proc Natl Acad Sci U S A, 92 (1995), 1525–1529.
- N. Yoshino, T. Ryu, M. Sugamata, T. Ihara, Y. Ami, K. Shi-nohara, et al., Direct detection of apoptotic cells in peripheral blood from highly pathogenic SHIV-inoculated monkey, BiochemBiophys Res Commun, 268 (2000), 868–874.
- E. Zuniga,˜ C. C. Motran, C. L. Montes, H. Yagita, and A. Gruppi, Trypanosoma cruzi infection selectively renders parasite-specific IgG+ B lymphocytes susceptible to Fas/Fas ligand-mediated fratricide, J Immunol, 168 (2002), 3965–3973.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14111
- [From(publication date):
December-2012 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 9592
- PDF downloads : 4519