Case Report Open Access
Cerebral Microsporidiosis Caused by Encephalitozoon cuniculi Infection in a Young Squirrel Monkey
K. Furuya1*, H. Sugiyama1, M. Ohta2, S. Nakamura2, Y. Une2, and S. Sasaki3
1Department of Parasitology, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162-8640, Japan
2Laboratory of Veterinary Pathology, Azabu University, 1-17-71 Fuchinobe, Sagamihara, Kanagawa 229-8501, Japan
3Kashima Laboratory, Mitsubishi Chemical Medience Corp., 14 Sunayama, Kamisu, Ibaraki 314-0255, Japan
- *Corresponding Author:
- K. Furuya
Department of Parasitology
National Institute of Infectious Diseases
1-23-1 Toyama, Shinjuku-ku
Tokyo 162-8640, Japan
E-mail: kfuruya@nih.go.jp
Received Date: 7 July 2011; Accepted Date: 19 July 2011
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
This is a case report of cerebral microsporidiosis found in a young squirrel monkey (Saimiri sciureus) in a colony located in Japan, which probably died of yersiniosis due to Yersinia pseudotuberculosis infection. The microsporidia, Encephalitozoon cuniculi, was detected in the brain of the yersiniosis-diseased monkey and was further characterized as a genetically unique type of strain III. The agent was microbiologically and genetically undetectable in other organs tested. Gram-positive organisms, which were confirmed immunohistochemically as Encephalitozoon spp. including mature spores, were histologically detected in pseudocysts formed inside neurons and in neuropils. Reactions in the surrounding tissue were not observed for most parasitized lesions. Neurons in the brain of younger hosts might provide a site for latent and active infection by E. cuniculi.
This is a case report of cerebral microsporidiosis found in a young squirrel monkey (Saimiri sciureus) in a colony located in Japan, which probably died of yersin-iosis due to Yersinia pseudotuberculosis infection. The microsporidia, Encephalitozoon cuniculi, was detected in the brain of the yersiniosis-diseased monkey and was further characterized as a genetically unique type of strain III. The agent was microbiologically and genetically undetectable in other organs tested. Gram-positive organisms, which were confirmed immunohistochemically as Encephalitozoon spp. including mature spores, were histologically detectedin pseudocysts formed inside neurons and in neuropils. Reactions in the surrounding tissue were not observed for most parasitized lesions. Neurons in the brain of younger hosts might provide a site for latent and active infection by E. cuniculi.
Keywords
Encephalitozoon cuniculi; cerebral micro-sporidiosis; squirrel monkey; genotype; latent infection
Microsporidian Encephalitozoon cuniculi infection in squirrel monkeys has been previously reported [14], where many reported cases were histologically diagnosed. Seropositive cases were also found in many squirrel monkeys (Saimiri sciureus) in a primate colony in the United States [9] and also in 11 squirrel monkey colonies in Japan [7]. Therefore, there was a considerable interest in clarifying whether seropositive squirrel monkeys truly develop microsporidiosis or E. cuniculi infection. We investigated five squirrel monkeys (AZ1, AZ2, AZ3, AZ4 and AZ5) in one of the 11 colonies located in Japan and determined that two squirrel monkeys (AZ1 and AZ2) had evidence of microsporidiosis. In a previous report, we mainly studied animal AZ1 through genetic testing and diagnosed it with disseminated microsporidiosis due to infection by a genetically unique type of E. cuniculi strain III [2]. The present report describes animal AZ2 with cerebral microsporidiosis, based on microbiologic, genetic, serologic, and histological investigation, with emphasis on histological results. The remaining three squirrel monkeys (AZ3, AZ4 and AZ5) were also investigated to understand the infection level in each monkey, and sera from another 14 monkeys in the same colony were also used to understand the endemic prevalence within the colony.
In 2005, two squirrel monkeys (AZ1 and AZ2) died of unknown causes in a colony located in Japan. AZ1 was a 10-day-old squirrel monkey and the AZ2 was a young male squirrel monkey with undeveloped canine teeth and immature testes. The 10-day-old animal and the young animal were later diagnosed with disseminated microsporidiosis [2] and yersiniosis due to Yersinia pseudotuberculosis, respectively. Major organs (brain,lung, liver, spleen and kidney) were obtained from the two animals at autopsy in addition to those from the AZ3 (a pubescent one with developed canine teeth and penis but with an undeveloped inguinal canal), AZ4 (adult) and AZ5 (adult) that had been bred in the same colony. The AZ3, AZ4 and AZ5 were humanely sacrificed to perform the various tests. Serologic testing was carried out using spore-ELISA (enzyme-linked immunosorbent assay) to determine IgG antibody against the spore wall (SW) and antibody against the polar tube (PT) of the pathogen [8], using two-fold serial dilutions of sera from a total of 19 monkeys in the colony, starting with a dilution of 1:200. Antibody titers were expressed as the reciprocal of each serum endpoint dilution.
Microbiologic tests were performed using homogenized organ specimens, trypsinized, and washed with 0.25% sodium dodecyl sulfate (SDS) solution. SDS-insoluble pre-cipitates were fractionated by density gradient centrifuga-tion with Percoll (Amersham Biosciences, Uppsala) and the resultant sediments (RS) were subjected to light microscopic test using the Fungi-Fluor stain kit (Polysciences, Warring-ton, PA, USA) and also genetic analysis, as previously described [2]. Genetic analysis was performed using DNA samples separated from the RS specimens, as previously described [2]. The polymerase chain reaction (PCR) was performed using primer sets (5 -tcctagtaatagcggctgac-3 ; 5 -tttcactcgccgctactcag-3 ) for amplification of the internal transcribed spacer (ITS) region, primer sets (5 -gcagttccaggctactac-3 ; 5 -aggaactccggatgttcc-3 ) for amplification of the polar tube protein (PTP) coding region, and primer sets (5 -actgacaagtaccacatc-3 ; 5 - ttggactcacacattagg-3 ) for amplification of the spore wall protein 1 (SWP-1) coding region, as previously described [2, 13]. PCR products were further analyzed using direct DNA sequencing.
Histological analysis was performed using formalin-fixed and paraffin-embedded tissue sections of the brain of AZ2. Tissue sections were stained with hematoxylin and eosin (HE), Brown-Hopps, chromotrope and immunohis-tochemical methods [5,12]. In the immunohistochemical test, brain tissue sections were affixed to polylysine-coated microscope slides, deparaffinized and treated with L.A.B. solution (Liberate Antibody Binding Solution; Polysciences) to reactivate damaged antigens. Sections were reacted with biotin-conjugated and affinity-purified rabbit anti-E. cuniculi antibodies (primary antibody) and biotinylated goat anti-rabbit IgG solution (secondary antibody; Zymed Laboratories, South San Francisco, CA, USA), followed by ready-to-use streptavidin-peroxidase conjugate solution (Zymed Laboratories). Finally, slides were exposed to HistoMark True Blue (KPL, Gaithersburug, MD, USA) stain, as previously described [5].
The ELISA titer of AZ2 serum was determined to be 800 for anti-SW and 400 for anti-PT antibody. On the other hand, anti-SW and anti-PT antibody titers in sera of AZ3, AZ4 and AZ5 were estimated to be 1600-25200. These results are summarized in Table 1. ELISA antibody titers in sera of 13 out of the other 15 monkeys in the same colony ranged from 800 to 51200 for both anti-SW and anti-PT antibodies.
| Fungi-Fluor test1 | PCR test2 | Serologic test3 | ||||||||||
| Animal | Br | Lu | Li | Sp | Ki | Br | Lu | Li | Sp | Ki | anti-SW | anti-PT |
| AZ14 | + | + | + | + | + | + | + | + | + | + | ND | ND |
| AZ2 | + | − | − | − | − | + | − | − | − | − | 800 | 400 |
| AZ3 | − | − | − | − | − | − | − | − | − | − | 25600 | 12800 |
| AZ4 | − | − | − | − | − | − | − | − | − | − | 3200 | 3200 |
| AZ5 | − | − | − | − | − | − | − | − | − | − | 3200 | 1600 |
1 Fungi-Fluor test was performed to detect microsporidian spores in specimens from the brain (Br), lung (Lu), liver (Li), spleen (Sp) and Kidney (Ki). The − and + signs show to be negative and positive, respectively.
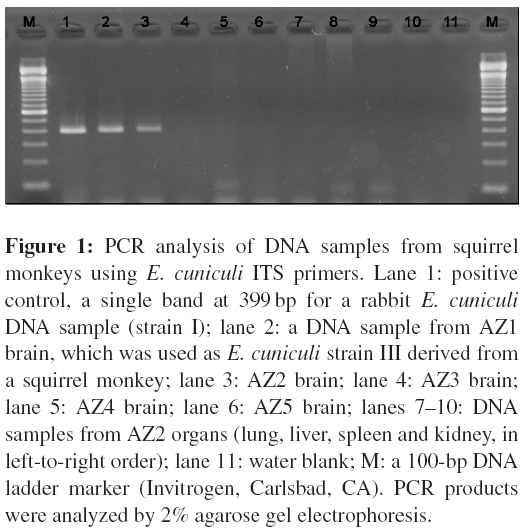
2 PCR test was performed to amplify E. cuniculi ITS region. As shown in Figure 1, a 403-bp fragment was amplified from the DNA sample from the brain of AZ2.
3 Serologic test was performed by spore-ELISA to detect Anti-SW and anti-PT IgG antibodies in the sera. The ELISA titer was expressed as the highest antibody dilution. ND: not done.
4 The results of AZ1 organs are described in detail in reference [2].
Table 1: Results of laboratory tests of organ specimens and serum samples from squirrel monkeys.
Fungi-Fluor-positive organisms were detected in the brain tissues of AZ2, but not from other organs tested from the same monkey or any organ tested for AZ3, AZ4 and AZ5 (Table 1).
Positive PCR tests targeting ITS, PTP and SWP-1 were detected only from DNA samples extracted from AZ2 brain tissue. ITS PCR amplification revealed a single band of 403 bp on agarose gels (Figure 1). DNA samples from the lung, liver, spleen and kidney were negative for the ITS-PCR. Furthermore, DNA samples from the brain and other organs of AZ3, AZ4 and AZ5 were ITS-PCR negative. These are also summarized in Table 1, in which the results of all the organs of AZ1 are also described for reference. Direct DNA sequencing of the PCR products demonstrated that the Encephalitozoon detected in AZ2 brain tissue was E. cuniculi ITS genotype III (i.e. strain III), because of thepresence of four repeats of 5 -GTTT-3 . However, further genetic analyses revealed that the detected Encephalitozoon had 99% homology with the corresponding region of the E. cuniculi genotype II PTP gene (GenBank accession:AF31068) and had the unclassified SWP-1 region (DDBJ accession: AB182699).
Figure 1: PCR analysis of DNA samples from squirrel monkeys using E. cuniculi ITS primers. Lane 1: positive control, a single band at 399 bp for a rabbit E. cuniculi DNA sample (strain I); lane 2: a DNA sample from AZ1 brain, which was used as E. cuniculi strain III derived from a squirrel monkey; lane 3: AZ2 brain; lane 4: AZ3 brain; lane 5: AZ4 brain; lane 6: AZ5 brain; lanes 7–10: DNA samples from AZ2 organs (lung, liver, spleen and kidney, in left-to-right order); lane 11: water blank; M: a 100-bp DNA ladder marker (Invitrogen, Carlsbad, CA). PCR products were analyzed by 2% agarose gel electrophoresis.
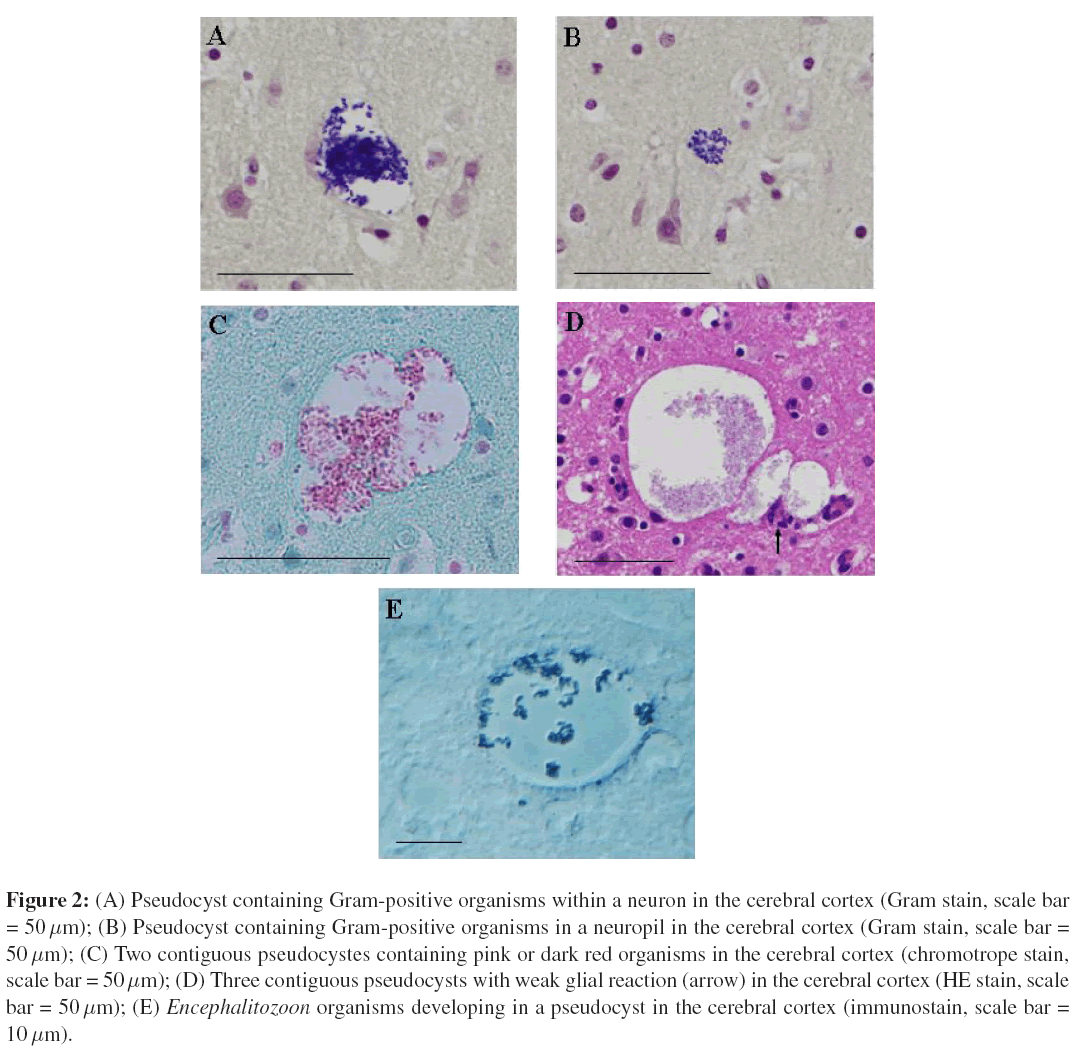
Histological evaluation of the brain of AZ2 was conducted on a transverse section of the telencephalon at the level of the thalamus. The histological characteristics of this case were non-suppurative meningoencephalitis, congestion, and fresh hemorrhage. Microscopic lesions in the brain consisted of multifocal microgliosis commonly related to small blood vessels, minimal perivascular cuffing with mononuclear cell infiltration, and partly adventitial cell proliferation. Gram-positive organisms were detected in pseudocysts formed inside neurons (Figure 2A) and in neuropils (Figure 2B). Parasitic organisms in the pseudocysts were also stained pink or dark red by chromotrope staining (Figure 2C). Most pseudocysts had no surrounding tissue reaction. Weak glial reaction was rarely seen around large pseudocysts contiguous to each other (Figure 2D). Perivascular macrophages were shown to have phagocytosed Gram-negative rod-shaped bacteria. Moreover, growth of Gram-negative rod-shaped bacteria free from cells was seen in the lumen of the blood vessels and in the hemorrhagic focus. Gram-positive organisms were confirmed as Encephalitozoon spp. developing inside pseudocysts (Figure 2E), by immunohistochemical staining with biotinylated and affinity-purified rabbit anti-E. cuniculi antibody. The HistoMark True Blue substrate deeply stained spores blue that also reacted with specific antibody.
Figure 2: (A) Pseudocyst containing Gram-positive organisms within a neuron in the cerebral cortex (Gram stain, scale bar = 50μm); (B) Pseudocyst containing Gram-positive organisms in a neuropil in the cerebral cortex (Gram stain, scale bar = 50 μm); (C) Two contiguous pseudocystes containing pink or dark red organisms in the cerebral cortex (chromotrope stain, scale bar = 50 μm); (D) Three contiguous pseudocysts with weak glial reaction (arrow) in the cerebral cortex (HE stain, scale bar = 50 μm); (E) Encephalitozoon organisms developing in a pseudocyst in the cerebral cortex (immunostain, scale bar = 10 μm).
Thus, we detected Encephalitozoon spores and DNA in the brain of AZ2 showing a low level of antibody; however, these observations were not found in the brain and other tested organs of AZ3, AZ4 and AZ5 in the same colony despite strongly seropositivity. The genotype of E. cuniculi identified in AZ2 was the same as that fromthe AZ1, which was identified as a genetically unique type of strain III [2]. Serological investigation also indicated the endemicity of E. cuniculi in the colony. The results of these analyses suggest that this type of E. cuniculi was prevalent among monkeys in the colony, but replication of the agent may have been restricted within newborn or young monkeys. Similar findings have been reported by Zeman and Baskin [14], who studied a series of 22 cases with naturally occurring microsporidiosis in squirrel monkeys from a breeding colony. Most of those monkeys were young, including nine monkeys that were less than four weeks old, and were histologically diagnosed. Furthermore, Anver et al. [1] reported a stillborn, premature male squirrel monkey diagnosed with microsporidiosis as indicated by histologic lesions. Brown et al. [3] described infection in a one-month-old orphan male squirrel monkey that had histologically suspected lesions and Gram-positive organisms in various organs including the brain.
A strong relationship has been proven between E. cuni-culi genotype and host specificity [4]; for example, strain I was isolated from rabbits, strain II from mice and blue foxes, and strain III from domestic dogs, generally known as the “dog type” [11]. However, E. cuniculi strain III has been identified in squirrel monkeys in this and previous stud-ies [2] and does not belong to the “dog type” because it contained a genotype II PTP and an unclassified SWP-1 genotype when being classified according to the procedures performed by Xiao et al. [13]. While such a genomic dif-ference is epidemiologically important for considering the original host of this strain III, it also may have influenced the distribution of the parasite and lesions seen in natural E. cuniculi infections of squirrel monkeys in a colony in Japan.
With the exception of perivascular macrophages possessing Gram-negative rod-like organisms, growth of the Gram-negative organisms in the blood vessels and hemorrhagic focus may suggest a postmortem change. Histological observations (Figures 2A and 2B) clearly show that pseudocysts containing Gram-positive Encephalitozoon organisms occurred in neurons and in neuropils. However, the host response was not observed in most parasitized lesions, although large pseudocysts rarely accompanied a glial reaction (Figure 2D). Thus, it was likely that the pathogen was reproducing in the absence of a host cell response, although the influence of co-infection by Y. pseudotuberculosis on E. cuniculi latent infectionis unknown. The current investigation and most cases reported in the United States suggest that at younger ages, various organs including the brain provide a favorable environment for latent infection by E. cuniculi and for its replication. It seems probable that E. cuniculi in an immune-privileged site is capable of remaining undetected in host cells. It has been previously observed that E. cuniculi occasionally spontaneously infects primary tissuecultures [10]. Therefore, it would be no surprise that a neuron was one of the target cells for latent infection by this type of strain III, as demonstrated in a study using mice experimentally infected with Toxoplasma gondii [6]. The present case is considered a representative example of cerebral microsporidiosis due to E. cuniculi strain III infection in young squirrel monkeys, and demonstrates histological features of latent infection.
Acknowledgment
We acknowledge Ms. T. Asakura for excellent technical assistance with immunoserologic, immunohistochemical, microbiologic and genetic analyses.
References
- M. R. Anver, N. W. King, and R. D. Hunt, Congenital encephalitozoonosis in a squirrel monkey (Saimiri sciureus), VetPathol, 9 (1972), 475–480.
- T. Asakura, S. Nakamura, M. Ohta, Y. Une, and K. Furuya, Genetically unique microsporidian Encephalitozoon cuniculi strain type III isolated from squirrel monkeys, Parasitol Int, 55(2006), 159–162.
- R. J. Brown, D. K. Hinkle, W. P. Trevethan, J. L. Kupper, and A. E. Mckee, Nosematosis in a squirrel monkey (Saimiri sciureus), J Med Primatol, 2 (1973), 114–123.
- E. S. Didier and G. T. Bessinger, Host-parasite relationships in microsporidiosis: animal models and immunology, in TheMicrosporidia and Microsporidiosis, M. Wittner and L. M. Weiss, eds., ASM Press, Washington, DC, 1999, 225–257.
- K. Furuya, Spore-forming microsporidian encephalitozoon: cur-rent understanding of infection and prevention in Japan, Jpn JInfect Dis, 62 (2009), 413–422.
- T. C. Melzer, H. J. Cranston, L. M. Weiss, and S. K. Halonen, Host cell preference of Toxoplasma gondii cysts in murine brain: A confocal study, J Neuroparasitol, 1 (2010), N100505.
- M. Ohta, Epidemiological and pathological studies on encephal-itozoon cuniculi infection in squirrel monkeys, master’s thesis,Azabu University, 2007 (in Japanese).
- M. Omura, K. Furuya, S. Kudo, W. Sugiura, and H. Azuma, Detecting immunoglobulin M antibodies against microsporidian Encephalitozoon cuniculi polar tubes in sera from healthy and human immunodeficiency virus-infected persons in Japan, ClinVaccine Immunol, 14 (2007), 168–172.
- J. A. Shadduck and G. Baskin, Serologic evidence of Encephali-tozoon cuniculi infection in a colony of squirrel monkeys (Saimiri sciureus), Lab Anim Sci, 39 (1989), 328–330.
- J. A. Shadduck and S. P. Pakes, Encephalitozoonosis (nosemato-sis) and toxoplasmosis, Am J Pathol, 64 (1971), 657–672.
- A. Tosoni, M. Nebuloni, A. Ferri, S. Bonetto, S. Antinori, Scaglia, et al., Disseminated microsporidiosis caused by Encephalitozoon cuniculi III (dog type) in an Italian AIDS patient: a retrospective study, Mod Pathol, 15 (2002), 577–583.
- R. Weber, D. A. Schwartz, and P. Deplazes, Laboratory diagnosis of microsporidiosis, in The Microsporidia and Microsporidiosis, M. Wittner and L. M. Weiss, eds., ASM Press, Washington, DC, 1999, 315–362.
- L. Xiao, L. Li, G. S. Visvesvara, H. Moura, E. S. Didier, and A. A. Lal, Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences, J Clin Microbiol, 39(2001), 2248–2253.
- D. H. Zeman and G. B. Baskin, Encephalitozoonosis in squirrel monkeys (Samiri sciureus), Vet Pathol, 22 (1985), 24–31.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 13183
- [From(publication date):
December-2011 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 8697
- PDF downloads : 4486