Research Article Open Access
Dopamine Hypothesis is linked with Neural Stem Cell (NSC) Dysfunction Hypothesis by D-Cell Hypothesis (Trace Amine Hypothesis) in Etiology of Schizophrenia
| Keiko Ikemoto* | |
| Department of Psychiatry, Iwaki Kyoritsu General Hospital, Iwaki 973-8555, Japan | |
| *Corresponding Author : | Keiko Ikemoto Department of Psychiatry Iwaki Kyoritsu General Hospital, Iwaki 973-8555, Japan E-mail: ikemoto@iwaki-kyoritsu.iwaki.fukushima.jp |
| Received December 16, 2014; Accepted March 04, 2015; Published March 11, 2015 | |
| Citation: Ikemoto K (2015) Dopamine Hypothesis is linked with Neural Stem Cell (NSC) Dysfunction Hypothesis by D-Cell Hypothesis (Trace Amine Hypothesis) in Etiology of Schizophrenia. Biochem Physiol 4:152. doi:10.4172/2168-9652.1000152 | |
| Copyright: © 2015 Ikemoto K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Mesolimbic dopamine (DA) hyperactivity is a well-known pathophysiological hypothesis of schizophrenia. The author intended to show a new hypothesis to clarify the molecular basis of mesolimbic DA hyperactivity of schizophrenia. The Patent Cooperation Treaty (PCT) patent-required histochemical methods were used to show D-neuron (trace amine (TA) neuron) decrease in the nucleus accumbens (Acc) of postmortem brains with schizophrenia. Briefly, the striatal D-neuron decrease in schizophrenia and consequent TAAR1 (TA-associated receptor, type 1) stimulation decrease onto terminals of midbrain ventral tegmental area (VTA) DA neurons induces mesolimbic DA hyperactivity of schizophrenia. Dysfunction of subventricular neural stem cells (NSC), located partially overlapping Acc is the cause of D-neuron decrease in Acc. DA hyperactivity, which inhibits NSC proliferation, causes disease progression of schizophrenia. The highlight is the rational that the “D-cell hypothesis (TA hypothesis) of schizophrenia” is a pivotal theory to link NSC dysfunction hypothesis to DA hypothesis. From a therapeutic direction, (1) TAAR1 agonists, (2) DA D2 antagonists, and (3) neurotropic substances have potential to normalize mesolimbic DA hyperactivity. To further develop novel therapeutic strategies, metabolisms of TAAR1ligands, and NSC- and D-neuron-pathophysiology of neuropsychiatric illnesses remain to be explored.
| Keywords |
| Dopamine; D-cell; Trace amine; Schizophrenia; TAAR1; Neural stem cell |
| Introduction |
| Dopamine (DA) dysfunction [1,2], glutamate dysfunction [3,4], neurodevelopmental deficits [5,6], or neural stem cell (NSC) dysfunction [7,8] are well-known hypotheses for etiology of schizophrenia. DA dysfunction hypothesis suggested that mesolimbic DA hyperactivity caused positive symptoms such as paranoid-hallucinatory state of schizophrenia [1,2]. It is also explained by the efficacy of DA D2 blockers for paranoid-hallucinatory state and also by hallucinogenic acts of DA stimulants including methamphetamine or amphetamine [1,2]. Glutamate dysfunction theory was induced by the fact that intake of phencyclidine (PCP), an antagonist of N-methyl-D-aspartate (NMDA) receptor, produces equivalent to negative symptoms of schizophrenia, such as withdrawal or flattened affect, as well as positive symptoms [3,4]. The neurodevelopmental deficits hypothesis implicates that schizophrenia is the consequence of prenatal abnormalities resulting from the interaction of genetic and environmental factors [5,6]. NSC dysfunction has also been shown to be a cause of schizophrenia [7,8]. Although mesolimbic DA hyperactivity [1,2] has been well documented in pathogenesis of schizophrenia, the molecular basis of this mechanism has not yet been detailed. In the present article, the author showed the rational of the reduction of putative trace amine (TA)-producing neurons (D-neurons), that is, ligand neurons of TA-associated receptor, type 1 (TAAR1), in the striatum in the pathogenesis of mesolimbic DA hyperactivity of schizophrenia [9]. The novel hypothesis, “D-cell hypothesis of schizophrenia”, is a critical theory to link NSC dysfunction hypothesis with DA hypothesis in etiology of schizophrenia. |
| D-neuron |
| The “D-cell” was described, by Jaeger et al. [10], in 1983 in the rat central nervous system and was defined “the non-monoaminergic aromatic L-amino acid decarboxylase (AADC)-containing cell”. AADC is an equivalent enzyme to dopadecarboxylase (DDC). The D-cell contains AADC but not dopaminergic nor serotonergic [10]. Then, it is natural that the D-cell is thought to produce TAs [11,12], such as β-phenylethylamine (PEA), tyramine, tryptamine and octopamine. AADC is the rate-limiting enzyme for TA synthesis. However, it is confusing that these TAs are also “monoamines”, as each one has one amino residue. It would be better to use the nomenclature of “TA cells” for D-cells, and “TA neurons” for D-neurons. In the present article, the author uses the words, D-cell and D-neuron, signifying TA cell and TA neuron, respectively. The localizations of D-cells were specified into 14 groups, from D1 (the spinal cord) to D14 (the bed nucleus of stria terminalis) in caudo-rostral orders of the rat central nervous system using AADC immunohistochemistry [13]. In this usage, the classification term “D” means decarboxylation. In rodents [14,15], a small number of D-cells in the striatum were rostrally described and confirmed to be neurons by electron-microscopic observation [14,15]. I reported in 1997, “dopa-decarboxylating neurons specific to the human striatum [16-19]”, that is, “D-neurons” in the human striatum [18,20] (classified to be D15) [18], though monkey striatum did not contain D-neurons [18]. In 2003, by using pathological and legal autopsy brains of patients with schizophrenia, reduction of D-neurons in the striatum, including nucleus accumbens (Acc) (classified to be D16) of patients with schizophrenia [9,20] was also shown. |
| Trace Amine (TA)-Associated Receptor, Type 1 (TAAR1) |
| Cloning of TA receptors in 2001 [21,22], elicited enormous efforts for exploring signal transduction of these G-protein coupled receptors whose genes are located on chromosome focus 6q23.1 [23]. The receptors have been shown to co-localize with DA or adrenaline transporters in monoamine neurons and to modulate the functions of monoamines [24-26]. The TAAR1 having a large number of ligands, including, PEA, tyramine, 3-iodothyronamine, 3-methoxytyramine, normetanephrine, and psychostimulants, for example methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA) and lysergic acid diethylamide (LSD) [21,23,26], has become a target receptor for exploring novel neuroleptics [27,28]. However, endogenous TAAR1 ligands in the human central nervous system have not yet been specified. TAAR1 knockout mice showed schizophrenia-like behaviors with a deficit in prepulse inhibition [29,30]. TAAR1 knockout mice showed greater locomotor response to amphetamine and released more DA (and noradrenaline) in response to amphetamine than wild type mice [29]. It has been shown that TAAR1 has a thermoregulatory function [30]. As is the important fact, it was clarified that increased stimulation of TAAR1 receptors on cell membranes of DA neurons in the midbrain ventral tegmental area (VTA) reduced firing frequency of VTA DA neurons [27-30]. This made the author to suspect the existence of critical role of TAAR1 stimulation decrease for mesolimbic DA hyperactivity in schizophrenia. |
| A New “D-Cell Hypothesis” of Schizophrenia |
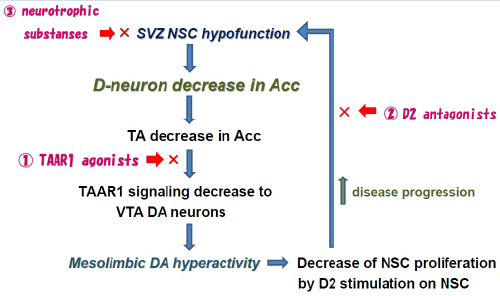
| A new theory, “D-cell hypothesis”, to explain mesolimbic DA hyperactivity in pathogenesis of schizophrenia is shown in Figure 1. In brains of patients with schizophrenia, dysfunction of NSC in the subventricular zone of lateral ventricle causes D-neuron decrease in the striatum and Acc [8,31]. This induces TA decrease in these nuclei, though direct evidences have not yet been demonstrated. Enlargement of the lateral ventricle [32,33], a usual finding documented in brain imaging studies of schizophrenia, is probably due to NSC dysfunction in the subventricular zone [7,8]. The reduction of TAAR1 stimulation on DA terminals of VTA DA neurons, caused by TA decrease, would increase firing frequency of VTA DA neurons [28,30,31]. This increases DA release and DA turnover in the Acc [2], resulting in mesolimbic DA hyperactivity. It has been shown that D2 stimulation of NSC in the striatum inhibited forebrain NSC proliferation [31,34]. Striatal DA hyperactivity may accelerate D-neuron decrease, which accelerates hyperactivity of mesolimbic DA system [35]. Actions of D2 blocking agents in pharmacotherapy of schizophrenia might be explained by blocking the inhibition to forebrain NSC proliferations, and also by formation of TAAR1 ligands, such as 3-methoxytyramine and normetanephrine [36]. It is consistent with clinical evidences that initial pharmacotherapy using D2 antagonists is proved to be critical for preventing progressive pathognomonic procedures of schizophrenia [35]. |
| Disease Progression of Schizophrenia and Therapeutic Strategies |
| D-cell hypothesis not only links DA hypothesis with NSC dysfunction hypothesis, but also explains the mechanisms of disease progression of schizophrenia as shown in Figure 1. To inhibit this cycle of pathological progression, intervention indicated by ΓΆΒ?Β ~ΓΆΒ?ΒΆ, shown in Figure 1, is supposed to be effective. |
| 1) TAAR1 agonists (Figure 1 ΓΆΒ?Β ) |
| Early studies have shown formation of some TAAR1 ligands by administration of D2 antagonists including haloperidol and chlorpromazine [35]. In recent animal studies, effectiveness of TAAR1 ligands for schizophrenia-like symptoms of schizophrenia model animals has been shown [28]. |
| 2) D2 antagonists (Figure 1 ΓΆΒ?Β΅) |
| Duration of untreated psychosis is a predictor of long-term outcome of schizophrenia [35]. Importance of early intervention for first episode schizophrenia by using D2 antagonist has been emphasized. Chronic D2 blocker administration has preventive effect for recurrence of psychoses. D2 antagonists may block disease progression as shown in Figure 1 ΓΆΒ?Β΅. D2 antagonists have dual actions for inhibiting this cycle of disease progression by also forming some TAAR1 ligands (3-methoxytyramine, normetanephrine) which may increase TAAR1 stimulation as shown in Figure 1 ΓΆΒ?Β [35]. |
| 3) Neurotrophic substances (Figure 1 ΓΆΒ?ΒΆ) |
| Disease progression would be inhibited by neurotrophic substances (Figure ΓΆΒ?ΒΆ), for example, brain-derived neurotrophic factor (BDNF), lithium, anticonvulsants, or antidepressants. These substances, having neurotrophic effects, activate NSC functions [37], and inhibit striatoaccumbal D-neuron decrease. |
| 4) Intranasal administration of drugs, expecting retrograde transport of neuroactive substances or their precursors through the olfactory bulb, might be a novel therapeutic strategy (ΓΆΒ?Β ~ΓΆΒ?ΒΆ). It is a possible preferable method of administration, as it devoid of gastrointestinal side effects [38-40]. In this context, further investigation remain to be performed. |
| Some Evidence Supporting D-Cell Hypothesis of Schizophrenia (Table 1) |
| Although it has not yet been detailed which type of TA in the human central nervous system is related to psychiatric symptoms, nor has been identified the endogenous ligands of human TAAR1, clinical and/or pharmacological observations may enable us to determine the critical type of TA. Further, the type of TA that is synthesized in human striatal D-neurons has not yet been clarified. Early in 1974, Sabelli and Mosnaim [41] proposed “Phenylethylamine hypothesis of affective behavior”, indicating the involvement of TA in animal behaviors. PEA, having the similar chemical structure of methamphetamine, is the most probable TA which effects on psychiatric symptoms. One of the initial clinical symptoms frequently observed in first episode schizophrenia is the disturbance of sleep-wake-rhythm, that is, insomnia and daytime hypersomnia. As PEA is the specific substrate for monoamine oxidase, type B (MAOB), MAOB knockout mice contained elevated level of PEA in the striatum by 8-10 times of that of controls [42]. Clinically, MAOB inhibitor, selegiline ameliorates daytime sleepiness of narcolepsy or other neuropsychiatric diseases. This is explained by PEA increase due to inhibition of PEA degradation by MAOB . The D-neuron decrease in the striatum of schizophrenia [9] due to NSC dysfunction causes striatal TA decrease. The author’s post-mortem brain study has shown increased DNA methylation rate of MAOB gene in Acc of schizophrenia [43]. This may be the compensation for PEA decrease caused by lack of D-neurons in Acc. From the aspect of food intake, PEA is included in chocolate. High incidence of chocolate habit of Novel Prizewinners, that is, eating chocolate more than twice a week, has been reported [44]. PEA is supposed to be related to higher mental functions. Whereas, too much chocolate intake of children is generally restricted, possibly aimed at preventing D-neuron down regulation. Carlsson and Lindqvist [36] reported that administration of D2 antagonists such as chlorpromazine and haloperidol increased TAAR1 ligands, including 3-methotytyramine and normetanephrine. This indicates that the molecular basis of efficacy of D2 antagonists may be effects also via TAAR1 stimulation by 3-methotytyramine and/ or normetanephrine. Ventricular enlargement in brain imaging of patients with schizophrenia [32,33] may be the similar phenomenon to D-neuron decrease in the striatum of schizophrenia [9], both of which support NSC dysfunction hypothesis of schizophrenia. Decreased level of plasma brain-derived neurotrophic factor (BDNF) in schizophrenia [40] is also related to NSC dysfunction. Some evidence supporting D-cell hypothesis of schizophrenia is summarized in Table 1. |
| Prognoses of Neuropsychiatric Illnesses |
| “D-cell hypothesis”, which is proposed by a postmortem brain study of schizophrenia, explains molecular mechanism of mesolimbic DA hyperactivity of schizophrenia, linking NSC dysfunction hypothesis with DA hypothesis. Such D-cell-involved etiological dynamism in schizophrenia may exist in wide spectrum of mental illnesses, and also in neurological illnesses [45]. As shown in Figure 1, NSC functions affect not only on D-neuron activity, but also clinical course and prognoses of neuropsychiatric illnesses. |
| Conclusion |
| The D-neuron, i.e., the TA neuron, is a clue for pathogenesis of neuropsychiatric illnesses. Exploration of endogenous TAAR1 ligands, and NSC- and D-neuron-mediated signal transduction of normal and/ or disease state(s) is critical for future direction of neuropsychiatric research. |
| Acknowledgement |
| The present study was supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (C-22591265, “How are trace amines involved in pathophysiology of schizophrenia?”). |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14201
- [From(publication date):
June-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 9617
- PDF downloads : 4584
