Research Article Open Access
L-Glutamine Therapy Reduces Hospitalization for Sickle Cell Anemia and Sickle β°-Thalassemia Patients at Six Months – A Phase II Randomized Trial
| Yutaka Niihara1, Henry Macan1, James R. Eckman2, Han Koh3, Melanie L Cooper2, Thomas R Ziegler2, Rafael Razon1, Kouichi R Tanaka1, Charles W Stark4* and Cage S Johnson4 | |
| 1Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, USA | |
| 2Grady Medical Center Atlanta GA, USA | |
| 3Kaiser Permanente Medical Center, Bellflower, CA, USA | |
| 4University of Southern California, Los Angeles, CA, USA | |
| Corresponding Author : | Charles W Stark University of Southern California Los Angeles, CA, 90501-1884, USA Tel: 310-214-0065 Fax: 310-214-0075 E-mail: cstark@emmausmedical.com, cstark@usc.edu |
| Received April 03, 2014; Accepted April 30, 2014; Published May 02, 2014 | |
| Citation: Niihara Y, Macan H, Eckman JR, Koh H, Cooper ML, et al. (2014) L-Glutamine Therapy Reduces Hospitalization for Sickle Cell Anemia and Sickle β°-Thalassemia Patients at Six Months – A Phase II Randomized Trial. Clin Pharmacol Biopharm 3:116. doi:10.4172/2167-065X.1000116 | |
| Copyright: © 2014 Niihara Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: Increased oxidant stress plays an important role in the pathophysiology of sickle cell disease. Nicotinamide Adenine Dinucleotide (NAD) is an important anti-oxidant that protects hemoglobin as demonstrated in diseases such as methemoglobinemia. Early in-vitro studies have shown that L-glutamine, a precursor for NAD, reduced oxidant stress via improvement of NAD redox status in red blood cells.Oral administration of L-glutamine in early clinical studies supported in-vitro findings of improving NAD redox potential, therefore, a larger proof of concept clinical trial was designed and conducted. Methods: A Phase II randomized, double-blind, placebo-controlled, parallel-group, multicenter study was conducted to evaluate the safety and efficacy of L-glutamine therapy for patients 5 years or older diagnosed with sickle cell anemia or sickle β°-thalassemia. Eighty one patients were randomized (1:1 ratio) to oral L-glutamine at 0.3 g/kg or placebo twice daily for 48 weeks. The primary endpoint was the frequency of painful crises. Secondary endpoints included the frequency of hospitalization. Results: At Week 24 (6 months), the mean number of painful crises was 2.5 and 5.5 for L-glutamine and placebo groups respectively (p = 0.060). The mean number of hospitalizations was 0.8 and 1.3 for L-glutamine and placebo groups respectively (p = 0.036). Conclusion: At 6 months of therapy, L-glutamine treatment was efficacious in reducing the frequency of hospitalization (nearly 40% reduction) and there was a major trend for the decrease in frequency of painful crises (over 50% reduction) favoring the L-glutamine treatment arm. There was no difference in safety between groups. Based on these findings, a Phase III trial was conducted and results are now available.
| Keywords |
| Treatment; Anemia; Glutamine |
| Introduction |
| Sickle cell disease is one of the most devastating hereditary disorders with severe pain and medical complications that can significantly impact quality of life and shorten life span. Progress has been slow since it’s molecular anomaly was first described in the 1940’s and only during the past couple of decades have data on polymerization of hemoglobin, increased endothelial adhesiveness, inflammatory processes, increased oxidant stress and other mechanisms leading to sickle cell syndrome been described [1-7]. Unfortunately, disease modifying therapies with minimal side effects have not been made available to date. |
| Glutamine is one of the most ubiquitous amino acids in the body in comparison to most other amino acids [8,9]. The efficacy of glutamine has been well studied under a number of human clinical conditions and determined to be useful [9-13]. L-glutamine is known for proven safety with no significant side effects [8-14]. |
| Since late 1980’s, our laboratory have focused on the process of increased oxidant stress in sickle red blood cells (RBC). Several studies have shown that the sickle RBC is more susceptible to oxidant damage than normal RBC [1-7]. Oxidant stress was described using changes in homeostasis of Nicotinamide Adenine Dinucleotide (NAD) molecules and it was named NAD redox potential [15-20]. Although NAD levels were found to be higher in sickle RBC, NAD redox potential was found to be consistently lower in sickle RBC by 20 to 30% compared to control. This suggested that sickle RBC was attempting to counter oxidant stress by producing more NAD molecules whereas overwhelming oxidant stress still caused a decrease in NAD redox potential [15-20]. |
| Subsequently, NAD metabolism in sickle RBC was carefully analyzed. It was observed that sickle RBC absorbed L-glutamine, a precursor for NAD, at significantly increased rates compared to control RBC, suggesting surrogate evidence that L-glutamine moved into sickle RBC to participate in NAD synthesis [21-24]. A hypothesis was formulated that with glutamine supplementation, increased transport and utilization of glutamine in sickle RBC may lead to an increase in NAD and NADH levels, thus, providing increased defense against sickle RBC oxidant stress. |
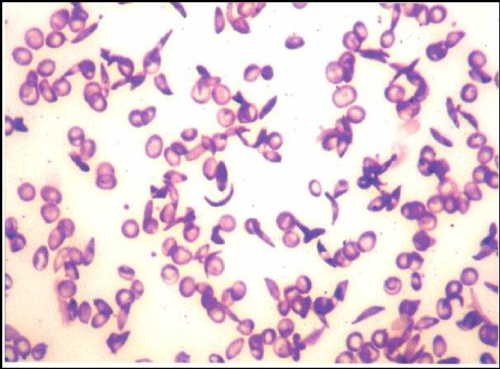
| This led to an open label pilot clinical trial to observe the effect of orally administered L-glutamine (30 grams daily*) on sickle RBC on NAD redox potential. The results were intriguing in that in every patient, NAD redox potential essentially normalized with reduction in subjective clinical symptoms [18,20]. In addition, there was a decrease in permanently sickled cells in the peripheral smear of room air incubated venous blood. Figure 1a* and 1b* illustrate the difference following 12 weeks of L-glutamine therapy. In another pilot study, we found a major decrease in endothelial adhesion rates when compared to controls [25-27]. These findings supported the rationale to design and conduct a multi-center phase II proof of concept clinical trial to examine L-glutamine therapy in comparison to placebo in sickle cell anemia patients. |
| *Data on file |
| Materials and Methods |
| This research was carried out according to the principles of the Declaration of Helsinki and in compliance with good clinical practice (GCP) and other applicable regulatory requirements. The study protocol was approved by the Institutional Review Boards (IRBs) of all participating sites: [1] Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California; [2] The Cancer Institute of New Jersey, New Brunswick, New Jersey; [3] Kaiser Permanente, Bellflower, California; and [4] Grady Memorial Hospital, Atlanta, Georgia. Signed informed consent or assent was obtained prior to the initiation of the patient in the study. This included discussion on the risks and/or discomforts that may be experienced by participants. This clinical trial was registered at http://www.clinicaltrials.gov. (Identifier: NCT00125788). |
| Criteria for eligibility were: at least 5 years of age; diagnosed with sickle cell anemia or sickle β°-thalassemia as documented by hemoglobin electrophoresis; had at least 2 episodes of painful crises within 12 months of the screening visit, if treated with an anti-sickling agent within 3 months of the screening visit, the therapy must have been continuous for at least 3 months with the intent to continue for the next 14 months; and, if female of childbearing potential, agreed to practice a recognized form of birth control during the course of the study. |
| Exclusion criteria included significant medical condition that required hospitalization (other than sickle painful crisis) within 2 months of the screening visit; diabetes mellitus with untreated fasting blood sugar >115 mg/dL; prothrombin time international normalized ratio (INR) >2.0; serum albumin <3.0 g/dL; received blood product within 3 weeks of the screening visit; treated with an experimental drug within 30 days of the screening visit; and, if in the judgment of the investigator, would have made it difficult for the patient to comply with the requirements of the study. |
| A patient could withdraw from the study at any time for any reason. In addition, an investigator could withdraw a patient if, in his/her judgment, it was in the patient’s best interest. All required evaluations for an early withdrawal were to be completed, and the reason for early withdrawal documented on the appropriate case report form (CRF). |
| Study design |
| This was a Phase II, randomized, double-blind, placebo-controlled, parallel-group, multicenter study to evaluate the safety and efficacy of oral L-glutamine therapy for patients with sickle cell anemia (homozygous SS) or sickle β°-thalassemia who were at least 5 years old. Randomization of approximately 80 patients was planned. Four weeks following the screening visit, eligible patients underwent baseline evaluations at Week 0. Patients who continued to meet all of the eligibility criteria were randomized (in a 1:1 ratio) to oral L-glutamine at 0.3 grams per kilogram (rounded to nearest 10, 20, or 30 grams per day) or oral placebo (maltodextrin) twice daily for 48 weeks. Study visits occurred monthly. After 48 weeks of treatment patients were gradually tapered off study medication over a period of 3 weeks before returning for their final visit at Week 53, 2 weeks following the final dose for the study. |
| Study endpoints |
| The primary objective of the study was to evaluate the efficacy of oral L-glutamine in therapy of sickle cell anemia and sickle β°- thalassemia as evaluated by the frequency of painful sickle cell crises. |
| Secondary efficacy objectives were to assess the effect of oral L-glutamine on 1) frequency of hospitalizations for sickle cell pain, 2) frequency of emergency room visits for sickle cell pain, 3) number of days patients’ usual daily activities were interrupted due to sickle cell pain, 4) height and weight, 5) growth curve for patients less than 18 years of age, 6) hematological parameters, 7) narcotic usage, 8) alcohol and tobacco use, 9) pain level, 10) energy level, 11) patient activity level, 12) patient appetite, 13) subjective exercise tolerance, and 14) subjective quality of life. Secondary safety objective of Overall Safety was evaluated by adverse events (AEs), serious adverse events (SAEs), laboratory parameters and vital signs. |
| Study medication dosing |
| L-glutamine or Placebo was provided as a powder that was mixed with beverage or food immediately before ingestion orally twice a day. The dosage was based on body weight (0.3 grams per kilogram per dose) and was adjusted in increments of 5 grams with an upper limit of 30 g/day. Patients were given verbal instructions for self-administration of the study medication and written instructions were also included on the consent form. Patients were instructed that the study drug should be taken between 6 am and 9 am and again between 6 pm and 9 pm. They were also instructed to mix the powder immediately before ingestion with water or any non-heated beverage other than alcohol or with any non-heated food such as yogurt, applesauce, or cereal. |
| After 48 weeks of treatment the dose was gradually tapered to zero over 3 weeks to minimize the possibility of sudden onset of a sickle cell crisis. For patients who withdrew early from the study before Week 48, the 3-week tapering period was started at the time of withdrawal (unless the reason for the withdrawal was that the patient had not taken any study medication over the previous study interval or was pregnant). Those patients returned for an early withdrawal (final) visit 2 weeks after completion of tapering. |
| Data processing and statistical analysis |
| Criteria for Evaluation: |
| Efficacy: The primary efficacy endpoint was the number of painful sickle cell crises through Week 48 and prior to the start of taper. A painful sickle cell crisis was defined as a visit to a medical facility that lasted more than 4 hours for acute sickling-related pain that was treated with a parenterally administered narcotic (except for facilities in which only orally administered narcotics were used). Secondary efficacy variables were number of hospitalizations for sickle cell pain at Week 24 and 48, number of emergency room visits for sickle cell pain at Week 24 and 48, days usual activities were interrupted due to sickle cell pain, height, weight, growth curve (<18 years of age), hematologic parameters, narcotic usage, alcohol and tobacco use, pain level, energy level, activity level, appetite, subjective exercise tolerance, subject quality of life (RAND 36-item Health Survey and Peds QL Pediatric Quality of Life Questionnaires) |
| Safety: Safety analyses were performed on the safety population, with no imputation of missing values. Safety endpoints include incidence of adverse events (AEs), serious adverse events (SAEs), clinical laboratory results, and vital signs. |
| Statistical Methods: |
| Populations: The safety population included all patients who received at least one dose of study medication (N = 70). The full analysis dataset included all patients who received at least one dose of study medication and had been diagnosed with sickle cell anemia or sickle β°-thalassemia documented by hemoglobin electrophoresis and had at least 2 episodes of painful crises within 12 months prior to the screening visit (N = 62). Table 1 describes the Safety Population and other dataset profiles. |
| The treatment groups were compared with respect to the number of painful sickle cell crises, number of hospitalizations, and number of emergency room visits using a Cochran-Mantel-Haenszel test. This non-parametric method was used due to the unanticipated number of non-completers resulting in a substantial proportion of imputed data. The data were not normally distributed and there was no suitable transformation. Two imputation methods were used. For discontinued patients with less than 85 days on treatment, the number of crises, hospitalizations, and emergency room visits were imputed by the mean number for the completed patients of the same treatment group. For discontinued patients with 85 days or longer on treatment, the number of crises, hospitalizations, and emergency room visits at week 48 were imputed by patient according to their individual rate at the date of withdrawal. All imputed values were rounded up to the nearest whole integer. |
| Results |
| Between April 23, 2004 and May 29, 2008, 81 patients were enrolled at five centers. Of these patients, data for a total of 19 patients were not included (due to reasons described in Statistical Methods above) for a total of 62 evaluable patients (Full Analysis Dataset) from four centers. Baseline characteristics between groups were comparable. The mean age was 30.5 and 26.5 years in the L-glutamine and placebo groups, respectively, with an overall range from 9 to 58 years. Six patients were under 18 years of age. The majority of patients in the L-glutamine group were female (66.7%) while the majority in the placebo group was male (65.5%). In both groups, most patients were African American and had a diagnosis of sickle cell anemia (93.9 and 82.8% respectively). Table 2 summarizes the patient baseline demographics for the Phase II Study. |
| Efficacy |
| Efficacy sample size was N = 62 (Full Analysis Dataset). For the primary efficacy parameter, the mean number of painful crises was 2.5 and 5.5 for L-glutamine and placebo groups respectively at Week 24 (p = 0.060). The mean number of painful crises was 4.5 in the L-glutamine group and 10.8 in the placebo group through Week 48 (p = 0.076). Table 3 shows the mean number of events and SD for the associated groups for the primary endpoint. |
| For secondary endpoints, the mean number of hospitalizations for sickle cell pain was 0.8 in the L-glutamine group and 1.3 in the placebo group through Week 24 (p = 0.036). The mean number of hospitalizations through Week 48 was 1.5 in the L-glutamine group and 2.3 in the placebo group (p = 0.072). Table 4 shows the mean number of hospitalizations and SD for the associated groups for the secondary endpoint. The mean number of emergency room visits for sickle cell pain was 3.7 in the L-glutamine group and 9.4 in the placebo group through Week 24 (p = 0.105) and 1.9 and 4.7 respectively through Week 48 (p = 0.129). |
| There were no notable changes in height or weight in either group or in alcohol or tobacco usage, although the majority of patients did not use either substance. Energy level tended to increase in both groups and there were no statistically significant between-group differences. A higher proportion of patients in the placebo group than the L-glutamine group had above average activity levels at Week 16 and above average appetite at Weeks 0 and 16; however, there were no statistical differences. There were no statistically significant betweengroup differences in the measures of subjective exercise tolerance or on the RAND-36 survey. |
| Safety |
| Safety population sample size was N = 70. Patients reporting more than one adverse event were counted only once (patient counts, not frequency of event counts). There was a 24% higher withdrawal rate in the placebo group in the safety data set (19/37 with 51.4% in the L-glutamine group and 21/33 with 63.6% in the placebo group). For Safety, imputed values were not utilized for missing data to adjust for early patient withdrawal. |
| Adverse Events (AEs) were reported for 94.6% (35/37) of patients in the L-glutamine group and 90.9% (30/33) of patients in the placebo group.Serious adverse events (SAEs) were reported for 64.7% (24/37) of patients in the L-glutamine group and 63.6% (21/33) of patients in the placebo group. Few patients had AEs that were considered at least possibly related to the study medication (8.1% L-glutamine, 9.1% placebo). Most AEs were moderate or severe. As expected, the most common AE was sickle cell crisis, which was reported for 83.8% of patients in the L-glutamine group and 78.8% of patients in the placebo group. The most common SAE was sickle cell crisis (59.5% L-glutamine, 51.5% placebo). For the most part, there were no unexpected patterns in the incidents of adverse events. There were few events that appeared disproportionately high in the placebo group. These were gastroenteritis (5% L-glutamine, 15% placebo) and arthralgias (5% L-glutamine, 21% placebo). One L-glutamine-treated patient died during the study due to multi-organ failure unrelated to the study medication. One placebotreated patient discontinued the study due to an AE (pregnancy). There were no notable differences between the treatment groups in clinical laboratory evaluations. |
| Discussion |
| Today, the option for disease modifying treatment is very limited for most patients with sickle cell disease. Stem cell transplantation may offer definitive cure to a small number of patients, however, by en large, current treatments are geared toward alleviation of symptoms and mitigating complications rather than modifying the pathophysiology of the disease. There remains a need for a safe, effective, and easy to administer therapy to address one of more of the mechanisms involved in sickle cell pathophysiology. |
| Evidence is accumulating for L-glutamine in ameliorating the pathophysiology of sickle cell disease. The rational for the use of L-glutamine is based on previous studies that have demonstrated the effect of L-glutamine in the improvement of NAD redox potential. In our in vitro study, we found that glutamine transport was increased approximately three-fold in sickle RBC compared to high reticulocyte controls [21,22]. In addition, in our in vivo trial, glutamine supplementation has improved NAD redox potential with positive subjective clinical response [18]. |
| While this was an exploratory proof of concept study, there were trend indicators favoring those who were treated with L-glutamine. For the primary efficacy parameter, there was a tendency toward fewer painful sickle cell crises in the L-glutamine group compared to the placebo group. On average, when examining differences in means between groups at Week 24, over 50% decrease in the incidence of painful crises and almost 40% decrease in incidence of hospitalization were observed. These results were more than we had expected from this population. At Week 24, the incidence of hospitalization between L-glutamine and placebo groups was statistically different (p = 0.038), however, the statistical difference was not maintained up to Week 48 (p = 0.072). |
| A larger sample size may have been necessary for achieving and maintaining statistical difference between groups for the major endpoints of this study. Additionally, statistical power may have been affected by an unexpectedly high withdrawal rate. Over the course of the study, in the full analysis data set (N = 62), the withdrawal rate was 49% in study medication group and 62% in placebo group (average of over 50%). In general, sickle cell studies have been noted as one of the more difficult from a study subject retention perspective. Retention issues are not uncommon in long term clinical trials due to factors around changing conditions and perspectives of the patient (and family) [28]. Another significant contributing factor to a reduced sample size for analysis may have been treatment compliance-based withdrawal requirement incorporated into this trial design. Patients were withdrawn from study if <75% of study medication was consumed. This may have contributed to the unexpectedly high withdrawal rate especially in the placebo arm of the study. Utilization of imputed data for patients withdrawn led to further decrease in power. Based on experience gained from this study, and with the advice from the FDA, the compliance requirement-based withdrawal was removed from the Phase III study design and the sample size was increased to 230 to enhance analytical power. |
| Adverse Events (AEs) and Serious Adverse Events (SAEs) were reported relatively equally between L-glutamine and placebo treatment groups and were most commonly related to sickle cell crises. Only a small number of patients reported events that were considered to be at least possibly related to treatment medication and there was no difference between groups. There were no unexpected patterns in the incidents of adverse events. |
| In summary, at 6 months, L-glutamine therapy reduced the frequency of hospitalization and indicated a positive trend towards reducing the frequency of painful crises. At Week 48, trends favoring the L-glutamine arm were maintained for major endpoints. L-glutamine was well tolerated during the overall duration of the study of nearly one year. Safety analysis of study therapy, as evaluated by adverse events and laboratory results, indicated no differences between L-glutamine and placebo arms. L-glutamine therapy was easy to administer and the data suggested that a well-designed pivotal study was warranted. A 230-subject, multicenter, 2:1 randomized, placebo-controlled, doubleblind, Phase III trial was conducted and the final report from this study is under preparation. |
| Acknowledgement |
| This work was supported by the Food and Drug Administration Office of Orphan Products Development (FDA OPD-01 Grant No. R29 HL58640). |
| References |
References
- Hebbel RP, Eaton JW, Balasingam M, Steinberg MH (1982) Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest 70: 1253-1259.
- Jain SK, Shohet SB (1984) A novel phospholipid in irreversibly sickled cells: evidence for in vivoperoxidative membrane damage in sickle cell disease. Blood 63: 362-367.
- Das SK, Nair RC (1980) Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br J Haematol 44: 87-92.
- Asakura T, Minakata K, Adachi K, Russell MO, Schwartz E (1977) Denatured hemoglobin in sickle erythrocytes. J Clin Invest 59: 633-640.
- Chiu D, Lubin B, Shohet SB (1979) Erythrocyte membrane lipid reorganization during the sickling process. Br J Haematol 41: 223-234.
- Campwala HQ, Desforges JF (1982) Membrane-bound hemichrome in density-separated cohorts of normal (AA) and sickled (SS) cells. J Lab Clin Med 99: 25-28.
- Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH (1980) Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med 302: 992-995.
- Wernerman J, Hammarqvist F (1994) Clinical experiences with glutamine supplementation. Nutrition 10: 176-177.
- Shabert J, Ehrlich N (1994) The Ultimate Nutrient Glutamine: The Essential Nonessential Amino Acid. Garden City Park, New York, Avery Publishing Group.
- Smith RJ, Wilmore DW (1990) Glutamine nutrition and requirements. JPEN J Parenter Enteral Nutr 14: 94S-99S.
- Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortos K, et al. (1992) Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med 116: 821-828.
- Lowe DK, Benfell K, Smith RJ, Jacobs DO, Murawski B, et al. (1990) Safety of glutamine-enriched parenteral nutrient solutions in humans. Am J ClinNutr 52: 1101-1106.
- Hammarqvist F, Wernerman J, von der Decken A, Vinnars E (1990) Alanyl-glutamine counteracts the depletion of free glutamine and the postoperative decline in protein synthesis in skeletal muscle. Ann Surg 212: 637-644.
- Ziegler TR, Benfell K, Smith RJ, Young LS, Brown E, et al. (1990) Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr 14: 137S-146S.
- Zerez CR, Lachant NA, Lee SJ, Tanaka KR (1988) Decreased erythrocyte nicotinamide adenine dinucleotide redox potential and abnormal pyridine nucleotide content in sickle cell disease. Blood 71: 512-515.
- Niihara Y, Zerez CR, Akiyama DS, Tanaka KR (1995) Normalization of redox potential in sickle red blood cells by oral administration of L-glutamine. Blood 86: 652a.
- Niihara Y, Zerez CR, Akiyama DS, Tanaka KR (1996) Elevation of redox potential in sickle red blood cells by oral administration of L-glutamine. J Invest Med 44:109a.
- Niihara Y, Zerez CR, Akiyama DS, Tanaka KR (1996) Oral L-glutamine therapy in sickle cell anemia: Improvement of redox potential and positive subjective clinical response. J Invest Med 4:215a.
- Niihara Y, Zerez CR, Akiyama DS, Tanaka KR (1997) Improvement of redox potential in a sickle cell anemia patient with oral L-glutamine at 10 grams a day. J Invest Med 45:102a.
- Niihara Y, Zerez CR, Akiyama DS, Tanaka KR (1998) Oral L-glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am J Hematol 58: 117-121.
- Niihara Y, Zerez CR, Akiyama DS, and Tanaka KR (1994) Increased red cell glutamine availability in sickle cell anemia. I. Demonstration of a several fold increase in active glutamine transport in intact red cells. Blood 84:405a.
- Zerez CR, Niihara Y, Akiyama DS, Tanaka KR (1994) Increased red cell glutamine availability in sickle cell anemia.II. Evidence for higher affinity red cell glutamine transport and higher plasma glutamine concentration.Blood 84: 411a.
- Niihara Y, Zerez CR, Akiyama DS, and Tanaka KR (1995) Increased red cell glutamate in sickle cell disease: Evidence that increased glutamine availability is a mechanism for increased total NAD. J Invest Med 43:131a.
- Niihara Y, Zerez CR, Akiyama DS, Tanaka KR (1997) Increased red cell glutamine availability in sickle cell anemia: demonstration of increased active transport, affinity, and increased glutamate level in intact red cells. J Lab Clin Med 130: 83-90.
- Niihara Y, Shen YM, Akiyama DS, Kalra VK, Tanaka KR (1997)Oral L-glutamine therapy for sickle cell anemia:II.Decreased adhesiveness to human umbilical vein endothelial cells. Blood 90: 29b.
- Niihara Y, Matsui NM, Sunga MA, Magpayo J, Johnson CS, et al (2000) Oral L-glutamine therapy reduces adherence of sickle red blood cells of human umbilical vein endothelial cells. Blood 96: 595a.
- Niihara Y, Matsui NM, Shen YM, Akiyama DA, Johnson CS, et al. (2005) L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells. BMC Blood Disord 5: 4.
- Good M, Schuler L (1997) Subject retention in a controlled clinical trial. J AdvNurs 26: 351-355.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
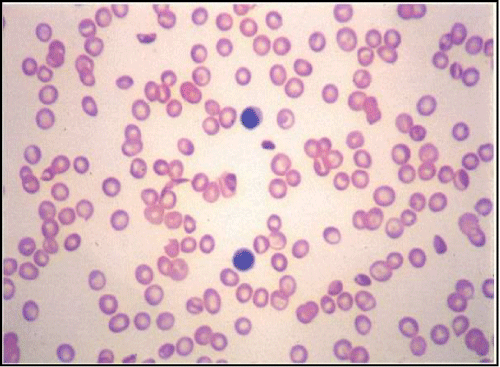 |
| Figure 1a | Figure 1b |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 19288
- [From(publication date):
June-2014 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 14187
- PDF downloads : 5101
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
