Research Article Open Access
Nitrergic Myenteric Neurons are Spared in Experimental Chagasic Megacolon
Mayra Fernanda Ricci1, Camila França Campos1, Christiane Teixeira Cartelle1, Samantha Ribeiro Béla1, Silvia Dantas Cangussú2, Helton da Costa Santiago3, Camila Megale de Almeida-Leite4 and Rosa Maria Esteves Arantes1*1Department of Pathology, Institute of Biological Sciences, Federal University of Minas Gerais, Brazil
2Departament of Biological Sciences ̸LAFEX, Federal University of Ouro Preto, Ouro Preto, Minas Gerais, Brazil
3Departament of Biochemistry and Immunology, Institute of Biological Sciences, Federal University of Minas Gerais, Brazil
4Departament of Morphology, Institute of Biological Sciences, Federal University of Minas Gerais, Brazil
- *Corresponding Author:
- Rosa Maria Esteves Arantes
Department of Pathology, Institute of Biological Sciences
Federal University of Minas Gerais
Av. Antonio Carlos, 6627, Pampulha
Belo Horizonte, Minas Gerais, Brazil
Tel: 3134092896
Fax: 31 3409-2879
E-mail: rosa.esteves.arantes@ufmg.br
Received date: November 23, 2016; Accepted date: December 21, 2016; Published date: December 23, 2016
Citation: Ricci MF, Campos CF, Cartelle CT, Béla SR, Cangussú SD, et al. (2016) Nitrergic Myenteric Neurons are Spared in Experimental Chagasic Megacolon. J Neuroinfect Dis 7:235. doi:10.4172/2314-7326.1000235
Copyright: © 2016 Ricci MF, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Chagas disease is a chronic disorder caused by the Trypanosoma cruzi protozoan. The infection causes alterations to the enteric nervous system such as megaesophagus and megacolon. There is evidence of denervation of myenteric ganglia. The intense parasitism of acute phase affects neuronal integrity but contrasts with the absence of parasites and the discreet inflammatory process of chronic phase, indicating a progressive injury mechanism that needs to be better understood in the megacolons. The potential selectivity of enteric neurons classes affected by the progression of the disease is not yet clear. Nitrergic neurons which co-localize other neurotransmitters represent the most common inhibitory neuron of the ENS. Recently a chronic stage of the Chagas disease was reproduced experimentally in a suitable murine model of megacolon. Considering the limitation of studying human intestine and the controversy on the pattern of nNOS involvement in chagasic megacolon, we decided to assess the nitrergic neurons in the myenteric plexus of mice. We used antibodies against structural protein gene product 9.5 (PGP 9.5) and functional neuronal nitric oxide synthase (n-NOS) at the acute and chronic phase of the disease to quantify myenteric ganglionar neurons in the colon of infected and non-infected mice. We found a reduction in the ganglionar number of neurons detected by anti-protein gene product 9.5 antibodies in colon from mice at the chronic stage. However, the number of nitrergic neurons per ganglia remained unchanged along the acute to phase chronic of the disease. Our findings indicate a long-term preservation of nitregic neurons detrimental to other classes of enteric in our model of experimental Chagas disease. We propose that differential loss of enteric neurons is at least one of the structural substrate for the development of the longterm morphfunctional changes that lead to the megacolon.
Keywords
Megacolon; Nitrergic neurons; Myenteric plexus; Neurodegeneration; T. cruzi ; Chagas´ disease
Introduction
Chagas disease (CD) is a chronic, neglected tropical disease in Latin America caused by the Trypanosoma cruzi protozoan and owing its high morbidity levels to the establishment of the chronic conditions. The infection by the parasite causes alterations in the enteric nervous system such as megaesophagus and megacolon. There is evidence of denervation of myenteric ganglia [1-5] as a pathogenic mechanism of intestinal accompaniment. The best chance to avoid morbidity is the treatment of the disease at its early acute stages [6] and novel therapeutic approaches are dependent in better understanding of the complex pathogenesis of this disease.
Megacolon is the chronic dilation of a colonic segment. Acquired enteric neurodegeneration of an originally healthy (normal ganglionic) segment is an important manifestation of at least 20% of patients suffering from Chagas’. During its chronic phase, myenteric neuron loss and irreversible dilation occur in the affected gut segment and the consequent dilation is termed as primary megacolon [7].
In patients suffering from Chagasic megacolon for many years, some ganglionic neurons are preserved intact while others are destructed which indicate that certain components of the ENS should be selectively undamaged, and also, implicates in the focal nature of the enteric plexus in this disease [8]. Indeed, recently we described focal accompaniment of the neuronal ganglia in the myenteric plexus of a murine model of long-term T. cruzi infection which reproduces chagasic megacolon [9].
In murine experimental models the mechanism related to degenerative processes in the acute phase has been studied and is directly related to the T. cruzi -induced immunoinflamation [10,11]. However, the mechanisms and potential selectivity of enteric neurons classes affected by the progression of the disease are not yet clear. The tissue infection and the intense parasitism of acute phase affect neuronal integrity [11,12]. However, the relative absence of parasite and the very discreet inflammatory process of chronic phase probably indicate a progressive injury mechanism, suggested to involve autoimmunity [7] which needs to be better understood in the context of the changes in TGI associated to the megacolons.
Significant neuronal loss is necessary to the development of human chagasic megacolon [1,4], and the different classes of neurons may be more or less affected [13-15].
The myenteric plexus displays excitatory and inhibitory neurons projecting to the muscle layers [16,17]. Among the different phenotypes of enteric neurons stands out the nitrergic neurons which co-localize other neurotransmitters, and represents the most common inhibitory neuron of the ENS. In many regions of the gastrointestinal tract the inhibitory reflex is required for the passage of fecal material and the final motor neurons of the reflex are enteric inhibitory, primarily nitrergic, the neuronal nitric oxide synthase positive (nNOS +) neurons [18]. Most human myenteric neurons are either nitrergic or cholinergic [19,20]. The primary neurotransmitter is Ach in the enteric excitatory muscle motor neuron [21-23], ascendant and descendants’ interneurons [21,24-29]. Sensitive neurons present ACh, CGRP e tachykinin as primary neurotransmitters [23,28,30,31].
The pattern of degeneration and survival of the myenteric neuronal populations may be associated to the attenuation of intestinal peristalsis [32], which, in turn, is physiologically involved in the development of the megacolon. For some authors, neuronal nitric oxide synthase (nNOS) neurons may be particularly susceptible in the presence of ganglionitis [33]. The chagasic megacolon has been associated with chronic constipation and inability of internal sphincter to relax, which is consistent with loss of nitrergic neurons which release nitric oxide NO via nNOS [34]. Also, a decreased expression of nNOS in myenteric plexus in experimental acute inflammation and aging contexts have been described [35,36].
In opposition, and in a contradictory direction research using human intestinal samples produced by the same group above cited revealed that chagasic megacolon display selective survival of inhibitory nitrergic neurons, which release nitric oxide NO via nNOS, and extensive reduction in cholinergic nerve fibre density expressing choline acetyltransferase (ChAT) [33,36,37]. Although one of these studies have addressed the differential loss of neuronal subclasses in human megacolon [7], the importance of reproducing this finding in a suitable model of murine megacolon is indiscutible and would provide a unique perspective to study the intrinsic mechanisms of the neural lesions in intestinal Chagas disease.
Considering the limitation of studying human intestine and the controversy on the pattern of nNOS involvement in chagasic megacolon, we decided to assess the nitrergic neurons in the myenteric plexus of mice at both the acute and chronic phases of CD. The profile of non-nitrergic neurons was accessed by neurokinin-1 receptor, choline acetyl transferase and vasoactive intestinal peptide were evaluated by RT-PCR as markers we used a suitable murine model of chagasic megacolon developed previously in our laboratory. In brief, we treated T. cruzi -infected mice at the acute phase of the disease with benzonidazole at sub-therapeutic doses [38] and reproduced the enlargement, the segmentary neuronal loss, and segmentary d axonal density decreasing in the muscular layer of intestine.
We hypothesized that the selective loss of non-nitrergic neurons arising from the inflammatory process triggered by the parasitic infection would result in long-term imbalance between excitatory and inhibitory neurons of the myenteric plexus. That would affect normal intestinal physiology, consequently leading to morpho-functional alterations characteristic of chagasic megacolon.
Materials and Methods
Animals
The mice were kept at the Universidade Federal de Ouro Preto animal facility in plastic cages in a temperature-controlled environment (25°C) under a 12:12 hour light/dark cycle. The animals had access to conventional mice feed (NUVILAB® Nuvital, Curitiba, Brasil) and filtered water ad libitum. They were treated against worms for seven days with 0.8 ml of IVOMEC® (Merial, Paulínia, Brasil) per litre of filtered water before the start of the experimental phase. All animals used in the research were acquired and cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals guidelines. All of the animal experiments were approved based on the regulations and guidelines of the ethical and animal use committee on animal experimentation (CETEA/UFMG 377/2012).
Infecting the mice with Trypanosoma cruzi
We used mice that developed Chagasic megacolon as described recently [38]. Briefly, parasite count for the preparation of the inoculum was performed according to the method described by Brener [38]. After positive confirmation of T. cruzi infection, the animals were divided into two groups: Infected acute phase group (IAP, n=10) composed of animals that were to be euthanized on the 11th day postinoculation thereby representing the infected group at the acute phase of the disease; and infected chronic phase group (ICP) whose animals were treated with a single sub-therapeutic dose of benzonidazole (ROCHAGAN® Roche, Rio de Janeiro, Brazil) at 500 mg/kg of body weight to ensure the survival of the experimental animals and, thereby, the chronicity of the disease. The treated mice that survived (20% of the infected and treated animals) did not show signals of sickness, pain or distress and were kept for 15 months, post-infection and denominated infected chronic phase group (ICP). In a pilot study, six non-treated mice were followed daily in order to obtain the necessary data required to build a parasitemia curve and determine the appropriate time for euthanasia. All experiments were performed twice.
Tissue sampling and preparation
Once the mice were euthanized at the time-points mentioned above, the entire colon was separated starting from the mesentery and washed in PBS (0.01 M phosphate buffered saline, pH 7.3). A segment of the colons collected was used for histological analysis. The colon samples used for histological analyses were stretched with the serosa in contact with the filter paper. All their contents were removed without damaging the mucosa and the segments were stretched on filter paper and prefixed with Bouin’s fluid containing 2.5% glacial acetic acid for 90 min to 120 min at room temperature. The prefixed segments were then rolled into a spiral with the mucosa facing inward, so as to form a “Swiss roll” similar to that described by por Calvert, Otsuka e Satchithanandam with adaptations by Arantes and Nogueira. The material was routinely processed for paraffin embedding and consecutive 4 μm thick histological sections were obtained [39,40].
Thirty-three paraffin blocks, containing the colons of the mice from each group, were selected as follows: eight blocks from the control acute phase (CAP) group; eight blocks from the infected acute phase (IAP) group; eight blocks from the control chronic phase (CCP) group and nine blocks from the infected chronic phase group (ICP). The previously HE cut non-serial sections from each sample were examined according to the histological criteria for: 1) the megacolon (thickening of the muscularis); 2) tissue inflammation (presence of mononuclear lymphocytes); 3) parasitism (presence of amastigotes); and 4) degenerative activity (presence of amastigote nests). Sections cut in series were used for immuno-labelling of specific proteins of interest.
Immunohistochemistry
Two swiss rolls histological sections (4 μm thick) from the colon were stained for PGP 9.5 (Cedarlane, USA) and for nNOS (Epitomics, USA) respectively by immunohistochemistry procedures. The slide was de-paraffinized in xylene and re-hydrated in decreasing ethanol concentrations. Endogenous peroxidase activity was blocked with 0.5% de H2O2 solution in methanol for 30 minutes. Unspecific binding was blocked with a 5% skimmed milk (MOLICO, Nestlé Brasil Ltda, Araçatuba, SP) in 10X PBS supplemented with 2% BSA (Bovine Serum Albumin, Inlab, Brasil) in 10X PBS, 10% FBS in 10X PBS (Fetal Bovine Serum-16140-071, Gibco, Invitrogen, Carlsbad, CA, USA), 1:20 NGS in 10X PBS (Normal Goat Serum, Cripton Biotechnology, Brazil). The slides were incubated for 30 minutes in each blocking solution and subsequently washed in 10X PBS after each incubation period. The slides were then incubated overnight at 4°C with the following rabbit anti-human polyclonal primary antibody anti-PGP 9.5 1:500 and rabbit monoclonal antibody anti-nNOS 1:750. The anti-nNOS used has specific bindging to PSD95, a neuronal membrane protein which is not present in other NOS isoforms (eNOS, iNOS) [41,42]. Additional sections were incubated with anti-T. cruzi antibody (1:5000, obtained from rabbits inoculated with blood trypomastigote forms of the Berenice-78 strain of T. cruzi provided by Dr. Maria Terezinha Bahia).
Once the incubation period with the primary antibodies was over, the slides were washed three times with PBS, followed by 30 minutes incubation with biotinylated secondary antibodies in a humid chamber at 37°C. Finally, the slides were incubated under the same conditions with streptavidin/peroxidase complex (Kit Dako, LSAB, K0675). Peroxidase activity was then detected using a 3.3 diaminobenzidine (DAB) substrate. All slides were counter stained with Harris hematoxylin.
We examined strictly consecutive sections immunostained for each antibody since the double immunostaining was prevented by the lack of primary antibodies raised in different species. PGP 9.5- and nNOSlabelled neurons were counted as their absolute number per ganglion (an average of 20 ganglions. nNOS-type neurons were confirmed by PGP9.5-labelling in the consecutive slides. Control reactions were perfomed by suppressing the primary antibody.
Photographic documentation and morphometric analysis
The slides were photographed using an Olympus B×51 (Japan) direct light optical microscope equipped with a Cool SNAP-Proof Color (Media Cybernetics, Bethesda, MD, USA) colored videorecording camera and Image-Pro Express 4.0 (Media Cybernetics, MD, USA) software. Eight mice colons from the CAP, CCP and IAP groups and nine from the ICP group were analyzed. To this end, 15 random images of the myenteric plexi covering the entire extension of the cut were captured with a resolution of 1392 × 1040 pixels. All visual analyses on images acquired using a 20X objective were performed using the freeware ImageJ 1.48. (version 1.47f, Wayne Rasband/ National Institutes of Health, USA) available online from the site http://rsbweb.nih.gov/ij/download.html
Only cells with characteristic morphology of neurons inside the ganglia were quantified when labelled to PGP 9.5 or nNOS. The average number of PGP+ and nNOS+ neurons, were counted per ganglion (16-21 ganglia/animal). The quantitative analysis was conducted at the Morphometrics Laboratory of the General Pathology Department at the Institute of Biological Sciences, UFMG.
Total RNA extraction
One annular sample measuring 1 cm was obtained from the proximal colon. Samples from each group (CAP n=5; IAP n=10; CCP n=3; and ICP n=6) were initially prepared for RNA extraction by adding 500 μl of TRIZOL (Trizol Reagent, Invitrogen #15596) and then homogenized with a homogenizer. Subsequently, 100 μl of chloroform were added to the samples, followed by a new round of homogenization for 15 seconds, incubation for 3 minutes followed by centrifugation at 12000XG for 15 minutes at 4°C.
The supernatant was removed and mixed with isopropanol, followed by incubation on ice for 15 minutes and centrifugation at 12000 × g for 15 minutes at 4°C. This time, the supernatant was discarded and the pellet washed with 75% ice-cold ethanol followed by vortex homogenization and centrifugation at 7500 × G for 5 minutes at 4°C. The pellet, dried by inversion, was re-suspended in 30μLof endonuclease-free water followed by drying at 55°C for 10 minutes. Quantification of the extracted material was performed on a Nanodrop (GE Nano Vue Plus Spectrometer) in ng Őłμl. The material was stored at -80°C until required.
cDNA synthesis
RNA reverse transcription into cDNA was performed in a total volume of 5 μl. To this end, 0.5 μL de Oligo dT 15 primer (Promega #C1101), 1 μL of RNA and 3.5 μL of endonuclease-free water were mixed for each reaction. The samples were vortexed for 30 seconds and placed in a thermocycler (PTC-100, MJ Research, Inc). The samples were firstly subjected to cycle at 70°C for 5 minutes and 40°C for 5 minutes after which the reactions were haulted for 5 minutes followed by the addition of 5 μL of a master mix containing: 0.3 μL of each nucleotide, 3 μl of buffer, 0.5 μL of reverse transcriptase (M-MLV RT, Promega, USA #M3682) and 0.25 μL of ribonuclease inhibitor. Transcription was allowed to resume at 42°C for 60 minutes followed by refrigeration at 4°C.
RT-PCR
We used 96-well plates to peform the RT-PCR experiments. Firstly, we dispensed 2 μL of sample and 13 μL of mix plus 7.5 μL of Power SYBR Green PCR Master Mix (Life Technology, England, 4267659), 0.2 μL of forward primer, 0.2 μL of reverse primer and 5 μL of endonuclease free water into each well. The amplification was performed using an Applied Biosystems, StepOnePlus Real-time PCR thermocycler. The cycling conditions used are as follows: an initial cycle at 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles of DNA-melting step at 95°C for 15 minutes. Primer annealing step at 60°C for 1 minute was followed by a final melting step. The primers used are as follows (Table 1).
| GAPDH | Forward | GACACCTTTGGCATTGTGG |
| Reverse | ATGCAGGGATGATGTTCTG) | |
| nNOS | Forward | ATGAAGTGACCAACCGCCTT |
| Reverse | AGCTGAAAACCTCATCTGTGTC | |
| ChAT | Forward | AGGGCAGCCTCTCTGTATGA |
| Reverse | ATCCTCGTTGGACGCCATTT | |
| VIP | Forward | GCAAGATGTGGGACAACCTC |
| Reverse | CAGTCTGTTGCTGCTCATCC | |
| NK-1 | Forward | GGTCTGACCGCAAAATCGAAC |
| Reverse | AGAGCCTTTAACAGGGCCAC |
Table 1: Analysis of the results was performed using the software StepOne v2.3. All the samples were analyzed in duplicate and normalized against the reference gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase). This gene is used as a constitutive control of the entire RT-PCR reaction. The values for 2-‚??‚??CT were expressed as the quantity of amplified gene in comparison to the reference gene.
Statistical Analyses
The statistical analyses were performed using the software GraphPad Prism (v 5.0) (GraphPad Software Inc., La Jolla, CA). For the immunohistochemical data we used Student’s t-test which compared the infected acute phase group (IAP) with the infected chronic phase group (ICP) and control chronic phase (CCP) and the infected chronic phase group (ICP).
RT-PCR results were analysed by Mann Whitney test according to data perform statistical comparisons between the groups. Differences were considered statistically significant if p ≤ 0.05.
Results
The chronic murine model of chagasic megacolon shows focal thickness of muscular layers, scarcity of parasites and persistent inflammation
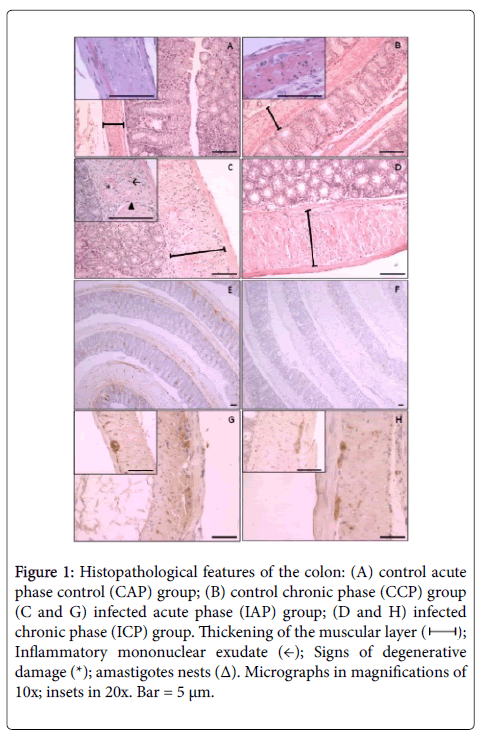
Compared to their control counterparts (Figure 1A), we observed a considerable thickening of the muscularis and submucosa in mice from the infected acute phase (IAP) group (Figure 1C) due to the edema between the muscle cells and the presence of inflammatory exudate which affects all intestinal layers. The inflammatory exudate, predominantly mononuclear, is distributed in foci along the entire extension of the histological cut forming ranks between smooth muscle cells or delineating and intercalating the myenteric plexi in the interior of the ganglia and close to their borders. Whole and ragged amastigotesnests were commonly observed in inflammatory foci (Figure 1C). We also observed a considerable thickening of the muscular wall due to volumetric hypertrophy of smooth muscle cells (Infected Chronic Phase, Figure 1D) compared to their control counterparts (Figure 1B). The inflammatory exudate foci were composed of mononuclear cells and were less frequent when compared to those from animals at the acute phase. Another exclusive characteristic of the chronic phase that we observed was the scarcity of T. cruzi amastigotes within the intestinal tissues (Figure 1E and 1F). The degenerative neuronal changes observed (Figure 1G and 1H) were intercalated with normal ganglionar aspects (Figure 1G and 1H, insets) that accounts for the focal histopathological accometiment of the colon.
Figure 1: Histopathological features of the colon: (A) control acute phase control (CAP) group; (B) control chronic phase (CCP) group (C and G) infected acute phase (IAP) group; (D and H) infected chronic phase (ICP) group. Thickening of the muscular layer ( ); Inflammatory mononuclear exudate (←); Signs of degenerative damage (*); amastigotes nests (Δ). Micrographs in magnifications of 10x; insets in 20x. Bar = 5 μm.
); Inflammatory mononuclear exudate (←); Signs of degenerative damage (*); amastigotes nests (Δ). Micrographs in magnifications of 10x; insets in 20x. Bar = 5 μm.
Megacolon at the chronic phase of CD is characterized by neuronal loss
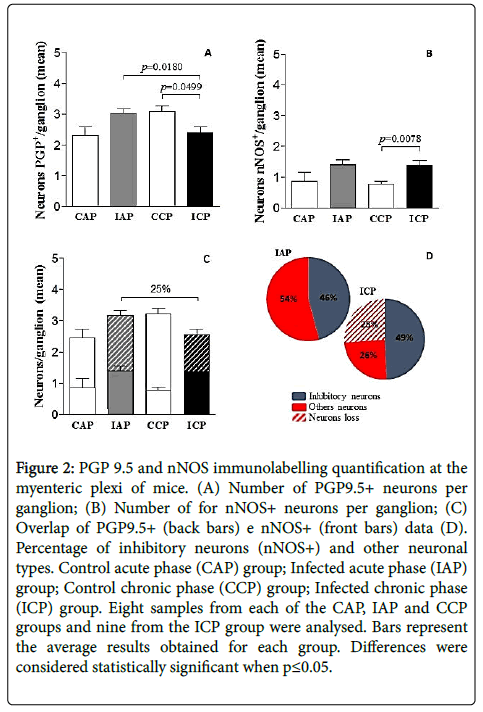
We evaluated the neuronal number per ganglia along the length of the colon (Figure 2A). There was statistically significant reduction in PGP9.5+ neurons from de group ICP versus its counter part control group (CCP) (p=0.0499). There was also a significant decrease of PGP9.5+ neurons from the ICP group compared to the group IAP (p=0.0180).
Figure 2: PGP 9.5 and nNOS immunolabelling quantification at the myenteric plexi of mice. (A) Number of PGP9.5+ neurons per ganglion; (B) Number of for nNOS+ neurons per ganglion; (C) Overlap of PGP9.5+ (back bars) e nNOS+ (front bars) data (D). Percentage of inhibitory neurons (nNOS+) and other neuronal types. Control acute phase (CAP) group; Infected acute phase (IAP) group; Control chronic phase (CCP) group; Infected chronic phase (ICP) group. Eight samples from each of the CAP, IAP and CCP groups and nine from the ICP group were analysed. Bars represent the average results obtained for each group. Differences were considered statistically significant when p≤0.05.
The average number of PGP9.5+ neurons per plexus was 24.59% fewer in infected animals at the chronic phase of the disease (ICP) compared to the acute phase of the disease (IAP) (Figure 2C). However, no significant differences were observed between the remaining experimental groups.
Nitrergic neurons are preserved in intestinal CD
Once we established that neuronal loss was a consequence of the chronic inflammation and the development of the megacolon is characterized by a reduction in PGP9.5 levels (as revealed by a fall in PGP9.5 by immunolabelling), we decided to investigate the role of nitrergic neurons in the neuronal rarefaction profile.
Analysis of the immunostaining experiment results against myenteric ganglia neurons’ nNOS showed a significant increase (p=0.0078) in the detection levels of this protein in the ICP group compared to its control (CCP group) counterpart when assessing the neuronal density per ganglia (Figure 2B and 2C). There were no statistically significant differences between the other experimental groups.
Interestingly, the number of nNOS+ neurons was maintained along the disease progression as observed by the data obtained from acute and chronic infected animals (Figure 2B and 2C). To better demonstrate this result, we overlapped PGP9.5 and nNOS data in order to evaluate the final distribution profile of nitrergic neurons along the disease phases. Thus, in Figure 3C we can observe the preservation of the nitrergic neurons profile representing 46% of the ganglionar neurons in both infected groups. On the other side, the neuronal loss seems to be restrict to non-nitrergic neurons when we compared the infected groups, from acute (54%) to chronic (29.4%) indicating an almost 25% ganglionar neuronal loss as indicated by PGP9.5, a generic neuronal marker (Figure 2D).
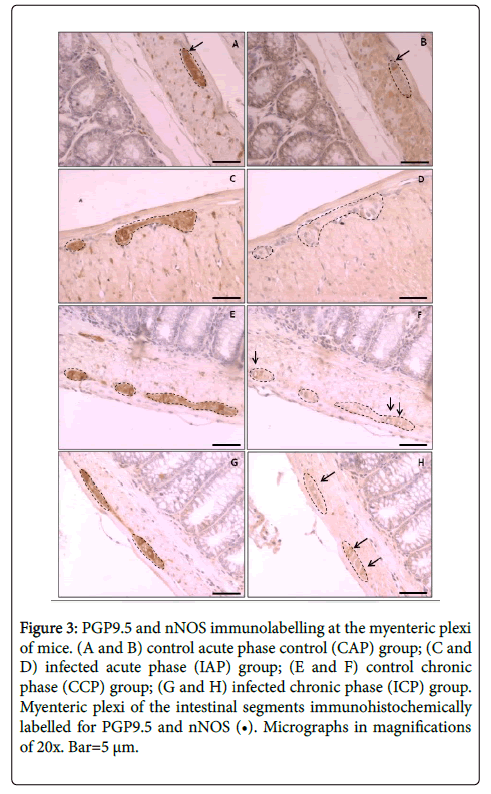
The consecutive colon segments containing ganglia with cells stained against PGP9.5 and nNOS were shown in Figure 3, and correspond to the aspects of the immunostaining of control and infected animals, as illustrated in acute phase (Figure 3A-3D) and chronic phase (Figure 3E-3H) of the disease.
Figure 3:PGP9.5 and nNOS immunolabelling at the myenteric plexi of mice. (A and B) control acute phase control (CAP) group; (C and D) infected acute phase (IAP) group; (E and F) control chronic phase (CCP) group; (G and H) infected chronic phase (ICP) group. Myenteric plexi of the intestinal segments immunohistochemically labelled for PGP9.5 and nNOS (â¬?¢). Micrographs in magnifications of 20x. Bar=5 μm.
A significant loss of excitatory neurons is linked to chronic infection and the development of the chagasic megacolon
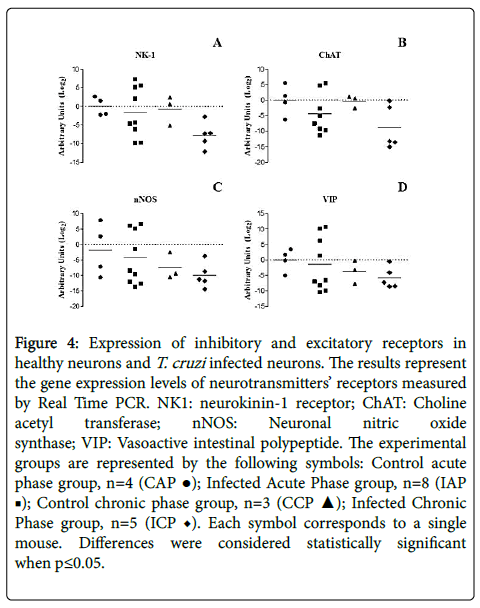
It is known that neuron proliferation is extremely difficult once fully differentiated: not only there are various types of neurons but also, their effective function is homogenetically distributed [43]. As such, the increase in number of nitrergic neurons observed during the chronic phase of the infection would be related to a possible loss of another class of neurons. To characterize the functional roles of the different neuronal types present in the colon as excitatory (NK1 and/or ChAT positive) or inhibitory (nNOS and/or VIP positive), we evaluated the gene expression profiles of the various kinds of neurotransmitters and their receptors using RT-PCR. Our data do not show a statistically significant change in any of the markers (Figure 4).
Figure 4:Expression of inhibitory and excitatory receptors in healthy neurons and T. cruzi infected neurons. The results represent the gene expression levels of neurotransmitters’ receptors measured by Real Time PCR. NK1: neurokinin-1 receptor; ChAT: Choline acetyl transferase; nNOS: Neuronal nitric oxide synthase; VIP: Vasoactive intestinal polypeptide. The experimental groups are represented by the following symbols: Control acute phase group, n=4 (CAP ‚?Ź); Infected Acute Phase group, n=8 (IAP ‚?†); Control chronic phase group, n=3 (CCP ‚?≤); Infected Chronic Phase group, n=5 (ICP ♦). Each symbol corresponds to a single mouse. Differences were considered statistically significant when p≤0.05.
Discussion
The pathogenesis of chronic intestinal CD and the development of the megacolon are of great importance due to the high levels of morbidity and disability generated by the condition, the difficulties in therapeutic approaches, and especially considering the utmost priority given to control and elimination of CD according to the 2020 London Declaration [6]. Furthermore, a comprehensive understanding of the mechanistic neurodegenerative processes involved in enteric denervation and its consequences such as dilatation, dysmotility and intestinal smooth muscle hyperplasia remains elusive. Since an appropriate nerve supply to intestinal smooth muscle fibres is an important factor in the proper functioning of gut contractility the intestinal inflammation [44], as well as the intrinsic and extrinsic neuronal alterations that affect intestinal smooth muscle can interfere in the motility of the gut. In the case of the chagasic megacolon, such acute and chronic inflammatory alterations may contribute to the progression of the disease.
Significant neuronal loss in the chronic phase of CD probably occurs due to the intense inflammatory process associated with the tissue parasitism that starts at the acute phase of the disease and turns into a consistent and long lasting chronic inflammation that affects segmentary the neuronal bodies and axons of the myenteric plexus. The neuronal loss observed in the current study could only be detected due to the extensive sampling technique described by Arantes and Nogueira [40], since the colonic acometiment is segmental.
Herein we report the total neuronal loss per ganglion and a paradoxal increase of nitrergic neurons (nNOS+/ganglion). This suggests this type of neuron is preserved in detriment of the loss of other neuron sub-types. It is known that nNOS neurons mediate most of the inhibitory responses in the gastrointestinal tract and regulate various important physiological reflexes such as the relaxation of the lower oesophageal sphincter after swallowing, receptive relaxation of the proximal stomach during feeding and descendent inhibition in response to distension [45]. Other studies, however, report a reduction in nNOS expression in the myenteric plexus during acute inflammation such as that observed in experimental models of colitis and aging [46,47] and human megacolon [34].
These studies propose that the inhibitory role for NO is lost due to the neuronal damage caused during the inflammatory process, leading to a decrease in inhibitory neuronal function and muscle fibre release for proliferation. However, our results using a new mouse model of chagasic megacolon [37] showed that nitrergic neurons are relatively more numerous and, as such, it is expected that their activity in the intestines prevails in the pathological state. Furthermore, we also showed recently that an increase in muscle fibre volume without evidence of hyperplasia is associated to the model development [37].
One should consider the limitation of human investigative studies includes small sampling of the intestine wall, especially because the inflammatory changes in intestinal Chagas disease are spotted. For example, a previous study using 4 chagasic patients with megacolon has shown that loss of cholinergic neurons leads to the selective survival of nitrergic neurons. Comparing nitrergic against cholinergic neurons in a human chagasic megacolon model, [36] demonstrated that nitrergic neurons were more numerous in lesioned ganglia. This suggests that such neurons are more resistant against the pathological factors that lead to neuronal loss. Selective preservation of enteric nitrergic neurons has also been observed as a consequence of selective loss of cholinergic neurons in chagasic patients [37].
Although other authors classified colonic myenteric neurons as “excitatory” (NK-1 and/or ChAT positive) or “inhibitory” (nNOS and/or VIP positive) we are aware of the simplification of this approach, since the idea of a balance between excitatory and inhibitory neurons [48-50], may be rejected by most modern authors [51]. Also, in the mouse colon, immunolabeling for the NK1-receptor has also been described in nitrergic neurons, and it has been stated that myenteric NK1R was mainly expressed by intrinsic primary afferent neurons [52]. In addition, this receptor has also been reported to be expressed by interstitial cells of Cajal and by the circular muscle. This may explain the absence of detectable differences seen in RT-PCR, especially because the tissue sampling was segmentary, while the immunostaining was performed in the entire colon extension.
In the current study, we used a murine model of chagasic megacolon developed by our group which presents structural alterations observed in the megacolon, the myenteric plexus and in intramuscular nerve terminals to investigate the root cause of the condition. The study revealed an alteration in the balance between nitrergic and no- nitrergic neurons, a possible consequence of the chronic inflammatory process triggered by the T. cruzi infection. Further investigation ont the mechanisms related to the nitrergic neurons resistance to the inflammatory damage is desirable.
Considering the extensive sampling method and despite limitations of our study we can suggest a functional role for the abnormal inhibitory neurotransmission in the genesis of motility disorders and in the structural alterations observed in chagasic megacolon reproduced in our murine model.
Conclusion
This is the first study showing that mice with a chronic chagasic infection present selective preservation of inhibitory nitrergic neurons, possibly explained by the loss of excitatory neurotransmitters. Our results expand the understanding of development of the chronic intestinal lesions incurred by CD in animal models. We are aware that injuries affecting neuronal network impact directly in organ functioning. If there is a resistance mechanism operating in distinct classes of enteric neurons during inflammatory process in general, it should be better investigated. Understanding the mechanisms resulting in preservation of neurons in Chagas´ disease may repercute in effective therapeutic approaches, not only because we could interfere preventing early neuronal damage, but also because this selective preservation may be related to the pathophysiology of the structural changes that account for the gut dilatation. Preventing acute or progressive neuronal loss and avoiding dysfunctional tissue remodeling are potential therapeutic strategies.
Acknowledgments
M.F.Ricci received a Msc scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil). C.T. Cartelle and S.R. Béla were funded by PNPD/CAPES grant 2248/2011. R.M.E Arantes received CNPq Research Fellowship, CNPq Grant 458832/2014-6 and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil) Grant PPM/2014 for financial support. C.M. Almeida-Leite received CNPq Grant 459228/2014-5.
References
- Koberle F (1968) Chagas’ disease and Chagas’ syndromes: The pathology of American Trypanosomiasis. Adv Parasitol 6:63-116.
- Fernandez A, Hontebeyrie M, Said G (1992) Autonomic neuropathy and immunological abnormalities in Chagas’ disease. Clin Auton Res 2:409-412.
- Adad SJ, Cançado CG, Etchebehere RM, Teixeira VP, Gomes UA, et al. (2001) Neuron count reevaluation in the myenteric plexus of chagasic megacolon after morphometric neuron analysis. Virchows Arch 438:254-258.
- Meneghelli UG (2004) Chagasic enteropathy. Rev Soc Bras Med Trop 37:252-60.
- Iantorno G, Bassotti G, Kogan Z, Lumi CM, Cabanne AM, et al. (2007) The enteric nervous system in chagasic and idiopathic megacolon. Am J Surg Pathol 31:460-468.
- Peterson JK, Bartsch SM, Lee BY, Dobson AP (2015) Broad patterns in domestic vector-borne Trypanosoma cruzi transmission dynamics: Synanthropic animals and vector control. Parasit Vectors 8:537.
- Jabari S, de Oliveira EC, Brehmer A, da Silveira ABM (2014) Chagasic megacolon: Enteric neurons and related structures. Histochem Cell Biol 142:235-244.
- da Silveira ABM, Arantes RME, Vago AR, Lemos EM, Adad SJ, et al. (2005) Comparative study of the presence of Trypanosoma cruzi kDNA, inflammation and denervation in chagasic patients with and without megaesophagus. Parasitology 131:627-634.
- Campos CF, Cangussú SD, Duz ALC, Cartelle CT, Noviello M de L, et al. (2016) Enteric neuronal damage, intramuscular denervation and smooth muscle phenotype changes as mechanisms of Chagasic megacolon: Evidence from a long-term murine model of tripanosoma cruzi infection. PLoS One 11:e0153038.
- Ben Younès-Chennoufi A, Hontebeyrie-Joskowicz M, Tricottet V, Eisen H, Reynes M, et al. (1988) persistence of Trypanosoma cruzi antigens in the inflammatory lesions of chronically infected mice. Trans R Soc Trop Med Hyg 82:77-83.
- Arantes RME, Marche HHF, Bahia MT, Cunha FQ, Rossi MA, et al. (2004) Interferon-gamma-induced nitric oxide causes intrinsic intestinal denervation in Trypanosoma cruzi-infected mice. Am J Pathol 164:1361-1368.
- Garcia SB, Paula JS, Giovannetti GS, Zenha F, Ramalho EM, et al. (1999) Nitric oxide is involved in the lesions of the peripheral autonomic neurons observed in the acute phase of experimental Trypanosoma cruzi infection. Exp Parasitol 93:191-197.
- Santer RM (1994) Survival of the population of NADPH-diaphorase stained myenteric neurons in the small intestine of aged rats. J Auton Nerv Syst 49:115-121.
- Phillips RJ, Kieffer EJ, Powley TL (2003) Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci 106:69-83.
- Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, et al. (2009) Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil 21:746-e46.
- Bornstein JC, Costa M, Grider JR (2004) Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil 1: 34-38.
- Furness JB, Callaghan BP, Rivera LR, Cho HJ (2014) The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv Exp Med Biol 817:39-71.
- Di Nardo G, Blandizzi C, Volta U, Colucci R, Stanghellini V, et al. (2008) Review article: Molecular, pathological and therapeutic features of human enteric neuropathies. Aliment Pharmacol Ther 28:25-42.
- Murphy EMA, Defontgalland D, Costa M, Brookes SJH, Wattchow DA (2007) Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil 19:126-134.
- Beck M, Schlabrakowski A, Schrödl F, Neuhuber W, Brehmer A (2009) ChAT and NOS in human myenteric neurons: Co-existence and co-absence. Cell Tissue Res 338:37-51.
- Brookes SJ, Steele PA, Costa M (1991) Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience 42:863-878.
- Holzer P, Holzer-Petsche U (1997) Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther 73:173-217.
- Grider JR (2003) Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther 307:460-467.
- Young HM, Furness JB, Povey JM (1995) Analysis of connections between nitric oxide synthase neurons in the myenteric plexus of the guinea-pig small intestine. J Neurocytol 24:257-263.
- Brookes SJ (2001) Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 262:58-70.
- Furness JB, Costa M (1982) Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: Their projections in the guinea-pig small intestine. Neuroscience 7:341-349.
- Monro RL, Bertrand PP, Bornstein JC (2002) ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol Motil 14:255-64.
- Gwynne RM, Bornstein JC (2007) Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol 5:1-17.
- Portbury AL, Pompolo S, Furness JB, Stebbing MJ, Kunze WA, et al. (1995) Cholinergic, somatostatin-immunoreactive interneurons in the guinea pig intestine: Morphology, ultrastructure, connections and projections. J Anat 187:303-321.
- Li ZS, Furness JB (1998) Immunohistochemical localisation of cholinergic markers in putative intrinsic primary afferent neurons of the guinea-pig small intestine. Cell Tissue Res 294:35-43.
- Johnson PJ, Bornstein JC (2004) Neurokinin-1 and -3 receptor blockade inhibits slow excitatory synaptic transmission in myenteric neurons and reveals slow inhibitory input. Neuroscience 126:137-147.
- Stojanoviń? M, Šń?epanoviń? L, Hrnńćiń? D, Rašiń?-Markoviń? A, Djuric D, et al. (2015) Multidisciplinary approach to nitric oxide signaling: Focus on the gastrointestinal and the central nervous system. Vojnosanit. Pregl 72:619-624.
- Rivera LR, Poole DP, Thacker M, Furness JB (2011) The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil 23:980-988.
- da Silveira ABM, D’Avila Reis D, de Oliveira EC, Neto SG, Luquetti AO, et al. (2007) Neurochemical coding of the enteric nervous system in chagasic patients with megacolon. Dig Dis Sci 52:2877-2883.
- Brehmer A, Lindig TM, Schrödl F, Neuhuber W, Ditterich D, et al. (2005) Morphology of enkephalin-immunoreactive myenteric neurons in the human gut. Histochem. Cell Biol 123:131-138.
- Jabari S, da Silveira ABM, de Oliveira EC, Neto SG, Quint K, et al. (2011) Partial, selective survival of nitrergic neurons in chagasic megacolon. Histochem Cell Biol 135:47-57.
- Jabari S, da Silveira ABM, de Oliveira EC, Quint K, Neuhuber W, et al. (2012) Preponderance of inhibitory versus excitatory intramuscular nerve fibres in human chagasic megacolon. Int J Colorectal Dis 27:1181-1189.
- Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo 4:389-396.
- Calvert RJ, Otsuka M, Satchithanandam S (1989) Consumption of raw potato starch alters intestinal function and colonic cell proliferation in the rat. J Nutr 119:1610-1616.
- Arantes RM, Nogueira AM (1997) Distribution of enteroglucagon- and peptide YY-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res 290:61-69.
- Dawson TM, Sasaki M, Gonzalez-Zulueta M, Dawson VL (1998) Regulation of neuronal nitric oxide synthase and identification of novel nitric oxide signaling pathways. Prog Brain Res 118:3-11.
- Watanabe Y, Song T, Sugimoto K, Horii M, Araki N, et al. (2003) Post-synaptic density-95 promotes calcium/calmodulin-dependent protein kinase II-mediated Ser847 phosphorylation of neuronal nitric oxide synthase. Biochem J 372:465-71.
- Sasselli V, Pachnis V, Burns AJ (2012) The enteric nervous system. Dev Biol 366:64-73.
- Blennerhassett MG, Lourenssen S (2000) Neural regulation of intestinal smooth muscle growth in vitro. Am J Physiol Gastrointest Liver Physiol 279:511-519.
- Brehmer A, Blaser B, Seitz G, Schrödl F, Neuhuber W (2004) Pattern of lipofuscin pigmentation in nitrergic and non-nitrergic, neurofilament immunoreactive myenteric neuron types of human small intestine. Histochem Cell Biol 121:13-20.
- Takahashi T, Qoubaitary A, Owyang C, Wiley JW (2000) Decreased expression of nitric oxide synthase in the colonic myenteric plexus of aged rats. Brain Res 883:15-21.
- Takahashi T (2003) Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol 38:421-430.
- Wilson AJ, Llewellyn-Smith IJ, Furness JB, Costa M (1987) The source of the nerve fibres forming the deep muscular and circular muscle plexuses in the small intestine of the guinea-pig. Cell Tissue Res 247:497-504.
- Uemura S, Hurley MR, Hutson JM, Chow CW (1998) Distributions of substance P- and VIP-immunoreactive nerve fibres in the colonic circular muscle in children. Pediatr Surg Int 14:66-70.
- Llewellyn-Smith IJ, Furness JB, Gibbins IL, Costa M (1988) Quantitative ultrastructural analysis of enkephalin-, substance P-, and VIP-immunoreactive nerve fibers in the circular muscle of the guinea pig small intestine. J Comp Neurol 272:139-148.
- Jänig W (2006) The integrative action of the autonomic nervous system. Neurobiology of Homeostasis. Cambridge Universty press, UK.
- Pelayo JC, Veldhuis NA, Eriksson EM, Bunnett NW, Poole DP (2014)Localisation and activation of the neurokinin 1 receptor in the enteric nervous system of the mouse distal colon. Cell Tissue Res 356:319-332.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 5147
- [From(publication date):
December-2016 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 4200
- PDF downloads : 947