Short Communication Open Access
Paradigm Shift in Transmission of Vector Borne Diseases
Upasana Shyamsunder Singh1, Monali Praharaj2, Chayan Sharma1 and Aparup Das1*1Division of Genomic Epidemiology, Centre for Research in Medical Entomology, 4 Sarojini Street, Chinna Chokkikulam, Madurai – 625002, India
2Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, 615 North Wolfe Street, Baltimore, MD 21205, USA
- *Corresponding Author:
- Aparup Das
Division of Genomic Epidemiology
Centre for Research in Medical Entomology
4 Sarojini Street, Chinna Chokkikulam
Madurai – 625002, India
Tel: +91 011 25307
E-mail: aparupdas.crme@gmail.com
Received date: October 21, 2016; Accepted date: November 10, 2016; Published date: November 20, 2016
Citation: Singh US, Praharaj M, Sharma C, Das A (2016) Paradigm Shift in Transmission of Vector Borne Diseases. J Emerg Infect Dis 1:116. doi: 10.4172/2472-4998.1000116
Copyright: © 2016 Singh US, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Disease and Pathology
Abstract
Infectious diseases transmitted by insect vectors, otherwise known as Vector Borne Disease (VBDs) are causing havoc in the tropical and sub-tropical regions of the globe. Diseases like malaria, dengue, chikungunya, and zika are endemic to many of the countries. These VBDs not only cause mortalities, but some of them also cause high morbidity. Under a traditional model of disease transmission, symptomatic human hosts (patients) are considered to be the reservoir of pathogens which are then taken by the insect vectors and infect a new uninfected human host. This model therefore, assumes that the rate of transmission of VBDs is dependent on the number of symptomatic hosts in the population. However, in recent years this model seems not to hold true. In turn, recent research works have indicated that disease transmission is majorly contributed by otherwise underestimated high number of asymptomatic individuals in the population. In this communication, we have brought about the cases of high number of asymptomatic individuals infected with malaria, dengue and chikungunya in synthesizing and bringing into notice the importance of asymptomatic infection and the future direction to control VBDs through appropriate surveillance. We have also proposed a model to understand the relationship between symptomatic/asymptomatic infections and outbreak of epidemic/ inter-epidemic periods.
Keywords
Vector borne diseases (VBDs); Malaria; Dengue; Chikungunya; Asymptomatic
Introduction
The vector borne diseases (VBDs), alone constitutes to around 17% of the estimated global burden of all human infectious diseases. Some of the major VBDs include Malaria, Dengue, Chikungunya, Schistosomiasis, Human African trypanosomiasis, Leishmaniasis, Chagas disease, Yellow fever, Japanese encephalitis, Tick-borne rickettsial diseases and Onchocerciasis. Insects that transmit these diseases include mosquitoes, ticks, and fleas which carry infective pathogens such as viruses, bacteria, and protozoa. These pathogens can be transferred from one host (carrier) to another via the insect vectors [1]. Importantly, countries that are most affected by the VBDs are also amongst the poorest countries in the world. In the past two decades, many important VBDs have re-emerged or extended to new parts of the world. Due to their capability to spread globally, changes in climatic conditions, altering ecologies and the increased migration of people and goods, an increasing risk of new or re-emerging VBDs in human as well as veterinary public health has come into sight [2]. The problems are mounted due to the fact that vaccines for majorities of the VBDs are not available and evolution and spread of drug resistant pathogens and insecticide resistant vectors have emerged for many leading VBDs.
Basic Mechanism of Transmission of VBDs
Malaria and dengue constitute the most deadly and the world’s fastest spreading vector-borne diseases, with an increase in disease incidences/ outbreaks over the last 50 years [1]. It is imperative that the success of the spread of a particular VBD depends on the effectiveness of its “transmission” from one to the other hosts. This “escape” mechanism of pathogens (microorganisms namely nematodes, protozoa, bacteria and viruses) from the host or reservoir of infection to transport to the new host are otherwise different in different diseases [3]. Transmission of VBDs can either be mechanical or biological via the vector. Mechanical transmission implies to basic transfer of the organism via superficially contaminated parts of the vector. Enteroviruses, protozoa and bacteria are some of the pathogens transmitted via the vector through faecal/ oral route. In this, no replicate or developmental change occurs in the arthropod. Biological transmission elucidates biological development of the pathogen that occurs in the vector. Biological transmission via vector can be propagative, cyclodevelopmental, cyclopropagative, vertical and direct transmission [4]. Arboviruses undergo propagative transmission, in which they restrict themselves only to multiply in tissues of vectors like mosquito, flies and ticks and are transferred to the host through infected saliva. Pathogens like Plasmodium undergo transmission by cyclo-propagative process. In this route of transfer, the microorganisms replicate and multiply in the vector along with undergoing change from one stage of development to the other. Transmission in which the pathogen curbs itself to undergo only development from one stage to another in the vector is characterised as cyclo-developmental type. Nematodes like Filarioidea show such dissemination pattern. Certain viruses like rickettsia rely on vertical transmission of itself via the vectors [5].
Different Models of Transmission of VBDs
Although vector control plays a vital role in preventing VBD outbreaks, in order to completely curtail the transmission of such diseases, one should understand the root cause of transmission. Various factors contribute to the spread (transmission) of VBDs. Even if there are huge number of vectors and virulent pathogen in the population, human susceptibility and type of susceptibility determines the rate of transmission of the VBD’s in the population [6]. According to the conventional model of transmission of VBDs, more the number of infected individuals in the populations, more should be the rate of transmission (Figure 1a). The determination of the infected individuals are usually dependent on symptomatic individuals, because they show symptoms which are related to a particular VBD [7]. Symptomatic host of VBDs are notably argued to have a higher transmission potential. For example in Leishmaniasis, a study has proved that hosts who present clinical manifestations are known to have higher parasite loads, in turn having higher transmission potential [8]. For chikungunya, it is evidenced by comparing the viremic profiles of symptomatic vs. asymptomatic host that disease transmission majorly happens through symptomatic individuals [9]. Similarly, studies on malaria mosquito infectivity levels in Thailand and Amazonian natives have resulted in high infectivity, and in turn, higher transmission potential of symptomatic cases to the vectors (as opposed to the asymptomatic cases) has been proposed [10].
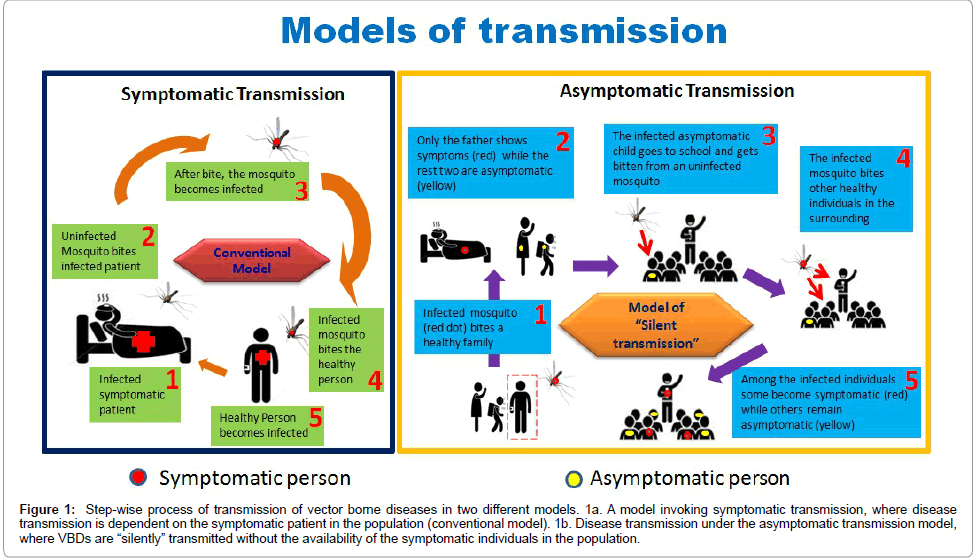
Figure 1: Step-wise process of transmission of vector borne diseases in two different models. 1a. A model invoking symptomatic transmission, where disease transmission is dependent on the symptomatic patient in the population (conventional model). 1b. Disease transmission under the asymptomatic transmission model, where VBDs are “silently” transmitted without the availability of the symptomatic individuals in the population.
In a traditional model invoking transmission of VBDs, when a particular symptom is not there in an individual (asymptomatic individuals), then they are not considered to be contributing to the disease transmission. However these “otherwise infected” individuals showing no symptom of the disease (asymptomatic) are not protected from bite of the vectors and vector can always be infected by biting these individuals (Figure 1b). Although it is very unclear that why some individuals do not show symptoms to a particular disease but off late, research work has shown that such asymptomatic individuals behave as the carriers and can transmit the disease [11]. This is supported by research works conducted in Nigeria, Gabon and Peruvian Amazon which have shown a positive correlation between higher transmissions potential and asymptomatic patients [12,13]. These observations opened up the possibilities that asymptomatic individuals might be the hiding sources for VBD transmission [14].
Silent Transmission of Malaria, Dengue and Chikungunya
In recent years, more number of asymptomatic cases is being reported from all over the VBD endemic countries with stable transmission of VBDs, supporting the statement that asymptomatic carriers, in fact are the keys for effective and “silent” transmission. Research works in three most widely prevalent VBDs (malaria, dengue and chikungunya) have provided preliminary evidences that transmission might be occurring mutely through the presence of asymptomatic individuals in the population.
• Evidences for quiet transmission in malaria:
About 3.2 billion people, i.e. almost half of the world’s population are at risk of malaria. Though there is a decrease in case incidences and mortality rates in recent years, but still in 2015, 95 countries and territories had ongoing malaria transmission. The probable reason might be resurgence of asymptomatic or undiagnosed malaria [15]. While in one hand, the World Health Organisation (WHO) has made a strategy which includes ambitious goals for malaria control and elimination in the next 15-year period (by 2030), on the other hand, almost all the endemic and holoendemic countries for malaria, e.g. Pakistan [16], Afghanistan [16], Uganda [17], Ghana [18], Colombia [19], Honduras [20], Nigeria [21], Ethiopia [22], Democratic republic of Congo [23], Cameroon [24], Kenya [25], Thailand [26], Gabon [27], Papua New Guinea [28], Brazil [29], Yemen [30], Bangladesh [31], Amazon [32], Solomon islands [33], Indonesia [34] report asymptomatic cases. To be noted that asymptomatic cases can interfere with malaria elimination strategies. In this regard, a great amount of work in India on malaria transmission indicates presence of both the most common malaria parasites in Indians (Plasmodium falciparum and P. vivax) both as single and also as mixed species infections, and transmission levels vary from high in the northeast with a predominance of P. falciparum to low in most of the country with a predominance of P. vivax [35]. A recent investigation in north eastern region of India reported, 45.31% of asymptomatic P. falciparum positive cases among all the asymptomatic cases [36]. Very similarly, a recent study carried out at Jimma town in Ethiopia reported that P. falciparum gametocyte carriage rate with asymptomatic Plasmodium infections was high (66.7%) as compared to P. vivax gametocyte carriage rate among asymptomatic P. vivax-infected individuals (12.9%) [22]. In an another study carried out at Arba Minch, the capital city of Ethiopia, donors with blood group O were found to be significantly more susceptible to asymptomatic malaria as compared to non-group-O donors [37]. Studies on the asymptomatic malaria infection in pregnant women in the Republic of Congo suggests that P. falciparum infections were associated with maternal anaemia, and use of IPTp-SP reduced the risk of carrying asymptomatic infections [38]. Subsequently it is also reported that febrile patients had stronger antibody responses than asymptomatic carriers [26]. All these case reports justify that asymptomatic malaria cases significantly contribute to transmission and such cases are continued to be coming up.
What causes the human subjects asymptomatic to malaria? It is debated that asymptomatic malaria infections might have resulted from partial immunity (sometimes referred to as “premunition”) that controls (but does not completely eliminate) malaria infection. It is sometimes believed that persistent or repeated “asymptomatic” infection is beneficial to the individual, as it helps to maintain this state of premunition, thereby reducing the risk of severe disease [39]. Therefore, asymptomatic malaria is possibly adaptive in nature for the parasites.
• Reports on hushed transmission in dengue:
Dengue, a major public health consequence and is the foremost arboviral infection emerging very fast in tropical and subtropical regions of the globe. Risk of dengue infections has evolved with an estimate of 390 million dengue infections per year [40]. Limiting factor for transmission of dengue is presence of vectors and their spatial distribution, signifying the epidemiological concern. Most important vectors of dengue are Aedes aegypti and Aedes albopictus. Studies suggest that the spatial distribution and infestations of both these vectors is influenced by climatic and ecological factors. Since Ae. aegypti is a dissonant species that requires more than one host to complete one blood meal, this is the reason why such habitats result in clustering of dengue cases in cities. In contrast, Ae. albopictus is a concordant species; it can complete its gonotropic cycle in one blood meal [41]. Dengue has been categorized into asymptomatic, pre-symptomatic (before the onset of disease) and symptomatic based on the clinical manifestations. In asymptomatic or unapparent infections, the presence of the virus is confirmed but existing surveillance systems are unable to detect the infection due of insufficient symptoms. Asymptomatic dengue cases have been reported in countries like Thailand [42,43], Central America [44], Singapore [45] and The Netherlands [46]. Dengue transmission has been correlated by investigators in Vietnam with kinetics of viremia [47]. It is now believed that 75% of all dengue infections are asymptomatic [48]. Studies have highlighted a correlation between disease severity with magnitude of viremia of dengue virus [47,49,50]. A study was performed in 2015 in the town of Kampong Cham [48], Cambodia in a human population at a risk of dengue infection. This study was essentially devoted to test the assumption that individuals with asymptomatic infections are not contributing to transmission of dengue virus. Laboratory – raised mosquitoes were fed with blood from 181 people through direct and indirect feeding methods that had been in contact with hospitalised patients with dengue symptoms and had detectable plasma levels of dengue virus RNA. It was found that among the participants, 126 were symptomatic when the mosquito feeding took place, 42 developed symptoms after feeding and 13 were asymptomatic [48]. It is therefore apparent that despite having lower levels of virus, asymptomatic and pre-symptomatic individuals were about 10 and 5 times more likely to effectively transmit dengue virus to mosquitoes, respectively through direct feeding as compared to symptomatic people. Very similarly, in the Indian capital city, New Delhi, case study was carried out during the 2015 outbreak and it was hypothesized that there were large number of asymptomatic dengue infections as compared to symptomatic cases in the community. This study also claimed that 63% of all the primary and secondary infections were asymptomatic [51].
It has been long assumed that asymptomatic individuals to dengue are unable to reach sufficient viremia levels; they do not infect mosquitoes and have any effect on the transmission [41]. However, mild illness and low viremia levels have evidenced to cause epidemic transmission of dengue [52]. As control of dengue is restricted by the understanding of the virus transmission from human to mosquito, addressing this undying debate of transmission is critical.
Soft transmission in chikungunya
Chikungunya is another tropical vector borne disease that is emerging with uncommon outbreaks with geographic restrictions. This alphavirus from the family Togaviridae causes acute illness characterised by fever, rash, and incapacitating arthralgia. The incubation period for chikungunya in humans (also known as intrinsic incubation period) is around four days. Upon infection, some individuals develop symptoms and some become asymptomatic (with a 3:1 ratio). The infective stage on an average lasts for 7 days with a range from 4 to 11 days [53]. Asymptomatic or unapparent forms of chikungunya virus (CHIKV) were published during 1996-97 chikungunya outbreaks in Senegal [54]. Surveys have found that 3%-28% of people infected with CHIKV remained asymptomatic [55]. In 2015, Kumar and others56 had reported two male patients with fever of one day duration. The fever subsided on the second day and both of the patients did not show any chikungunya symptoms. However, blood drawn from these patients on the second day showed CHIKV positive by RT-PCR. It is manifested that depending upon the immune status both of these patients might have acted as asymptomatic carriers [56].
For reasons unknown, India seems to be a major centre of chikungunya infection. Initially, a major outbreak was prevalent in south India but now suddenly captured North India. This is evidenced by the fact that this year (2016) in India, sudden outbreak of chikungunya caused 2,625 cases with 12 deaths. During earlier Indian outbreak (2006) in which Ae. aegypti is the alleged vector, 1,400, 000 cases of chikungunya were reported. The reasons for the recurrence of chikungunya on the Indian subcontinent, and the distinctive incidence rate in India, are still unclear [57]. However, change in epidemiological patterns of chikungunya cases and an increase in number of deaths in India is a matter of major concern that needs to be given immediate attention.
Why Asymptomatic Transmission?
What has made the shift in paradigm of symptomatic to asymptomatic cases in malaria, dengue and chikungunya? Since these asymptomatic cases contribute highly to the transmission that happens in the background, this contributes to a large extent to the global disease burden. Clearly, mysteries involving such “silent transmission” have not yet been resolved. What factor(s) prompt the increase in the case of asymptomatic transmission is still not known, neither the organism involved in it is cornered. Based on the short generation time and therefore high mutability of the pathogens, it seems, pathogens might be the only modulator of such asymptomatic transmission. Basically, many pathogens do not aim for causing disease in the host [58]; they just want to use the host machinery for their survival and propagation as a parasite [59]. A successful parasite will never kill its host, as by doing this the pathogen kills itself. However in the process of carrying out an essential act for parasite survival and multiplication, it might cause certain physiological changes by which the host’s physiological machinery gets disturbed. Therefore, the host becomes diseased and ultimately the host dies; the infecting strain of the parasite is considered to be highly pathogenic. Since based on evolutionary principle that no living organism will make anything by which it will die, it seems, the trade-off between survival and multiplication rate gets distorted and therefore, to largely multiply, the parasite kills the host and in turn kills itself [60]. However, with time, the parasites “learn” how to adjust between “survival” and “multiplication” and try to survive utilizing host machinery as efficiently as possible, without stressing much on the multiplication [61]. This is the reason perhaps; more newly emerged VBDs cause initial havoc and with time the pathogens “learn” how to use the host machinery for its survival at the same time not killing the host. Therefore deaths in many of the older VBD are diminishing and number of symptomatic individuals has also decreased; at the same time, number of asymptomatic individuals has been increased. Additionally it might be true that the pathogens also probably have evolved to an extent to infect many hosts instead of infecting a single host in large number. So in a population the overall number of parasites is maintained but the load of parasite in a patient (parasitemia) remains low. The classical example of this kind of model comes from P. vivax which is considered to be much more evolved parasite as compared to P. falciparum. In recent years it seems that P. falciparum has taken the same route as cases of asymptomatic infections have started to be reported from endemic countries [62].
Evolutionary Correlations of Transmission and Epidemic In VBDs
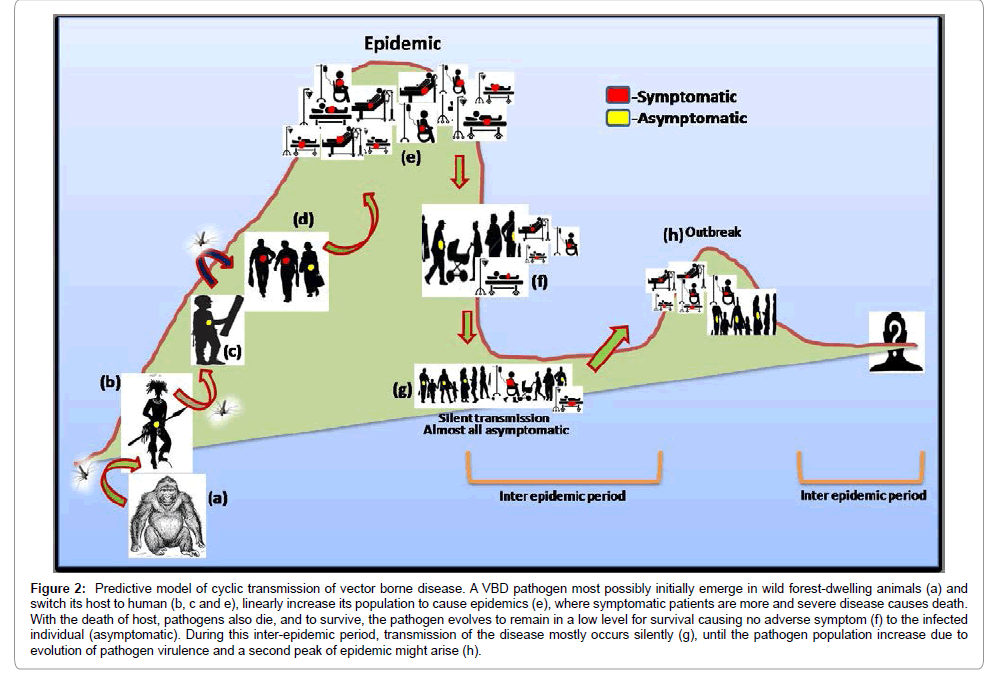
We often try to unravel the probable reasons for newly emerging diseases and the change in the scenario of transmission pattern of different VBDs [63]. This is apparent from the reports made mostly by all endemic and holoendemic countries about the asymptomatic cases exceeding the symptomatic ones. To understand any case it is important to know the prel0.iminary phase. Since a new VBD comes to light only after an outbreak (signifying the presence of many symptomatic individuals in the population), it is generally believed that symptomatic cases appear first and then appears the asymptomatic case. Whether the reverse is true is not known. Results from recent research on malaria suggest host switching events of malaria parasite, P. falciparum [64,65]. These sounds logical, as for diseases like malaria, JE and dengue the pathogens have sylvatic cycle. It is also true that in the race of evolution such tiny organisms (viruses, bacteria and protozoa) are par ahead of mammals specifically humans due to shorter generation time. That means these miniature organisms learn to survive in new hosts very quickly. Hence the hypothesis of “host switching” might be the first evolutionary step in the process of transmission of vector borne diseases [66,67]. Because it has recently entered a new host it might take some time to understand the host machinery before it can proliferate and expand its population size inside a host. In the process of learning the host machinery the pathogen becomes less pathogenic initially wherein it stays silently in the host (asymptomatic) (Figure 2b and 2c). Soon the pathogen learns the host (new) machinery; it makes an effort to increase its population size and in the process becomes more pathogenic to the host (symptomatic) (Figure 2d). This is when we became alert (epidemic situation) and put intervention measures in place (Figure 2e). These pathogens also understand that by increasing their number in a single host they are actually reducing their number in the population (more parasitemia causing death of human host). Therefore, in order to sustain their presence in the population they have to find some other way out. Here comes the second phase where the pathogens starts maintaining a silent status (Figure 2f) by evolving to a less “pathogenic” form that might cause asymptomatic disease manifestation and contribute to “silent transmission” (Figure 2g). In future these pathogens by getting high virulence might again lead to an outbreak (Figure 2h) and then again become silent. Therefore this kind of cyclic epidemic and inter-epidemic periods justify the maintenance of symptomatic and asymptomatic forms of the pathogen in the population. Tracking the silent zones of pathogens in the interepidemic period is important to end the malevolence. “Where, when and why these pathogens become silent” is the question of the hour which should be looked upon.
Figure 2: Predictive model of cyclic transmission of vector borne disease. A VBD pathogen most possibly initially emerge in wild forest-dwelling animals (a) and switch its host to human (b, c and e), linearly increase its population to cause epidemics (e), where symptomatic patients are more and severe disease causes death. With the death of host, pathogens also die, and to survive, the pathogen evolves to remain in a low level for survival causing no adverse symptom (f) to the infected individual (asymptomatic). During this inter-epidemic period, transmission of the disease mostly occurs silently (g), until the pathogen population increase due to evolution of pathogen virulence and a second peak of epidemic might arise (h).
What Needs to be Done Now?
The principal problem in having asymptomatic cases of infection is that since such cases arise without obvious symptoms, these are therefore invisible to the health system and do not come to clinical attention - thus representing a large hidden reservoir of active infection that allows their persistence and ultimately spread to their human hosts [68]. Asymptomatic cases have also been suggested to contribute the persistence of transmission in low transmission settings [69]. Clearly when asymptomatic individuals are there in the population no intervening measures are possible. This brings the disease to be circulated silently without the knowledge of the infected individual so that no chemotherapic or intervening measures can be taken to stop transmission [1].
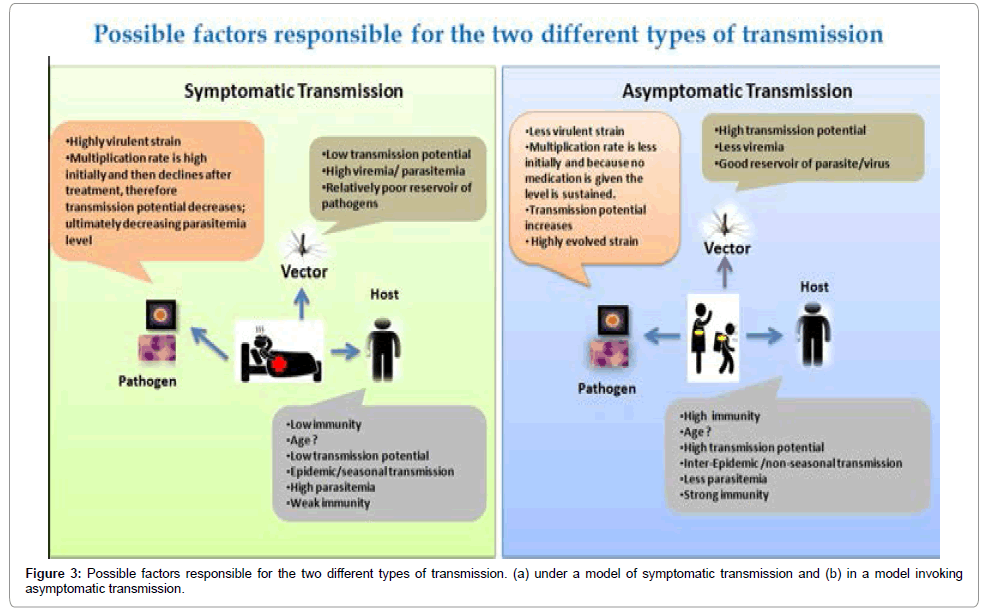
The only method by which intervening measures for a particular VBD can be taken, is by putting a concrete and planned surveillance mechanism into actions in areas where even past outbreak/incidences of a particular disease is not taken. Such effective surveillance will unfold the mystery of silent transmission and will stop future outbreak of the disease [70]. In depth vector surveillance includes the major part of disease surveillance mechanism since different pathogens are also invading new vectors (Zika virus is now infecting Culex mosquitoes in addition to Aedes), making identification of pathogens from mosquitoes necessary. Surveillance system should be very regional, focal and local. Such kind of focal surveillance should be used to develop a particular model and this model can be spread to the country setting [71]. Comprehending the factors contributing to asymptomatic carriage of malaria infection will help us to forecast where, when and why the asymptomatic cases are most likely to occur. Determinants of asymptomatic infections are (i) pyrogenic thresholds in host (humans), (ii) multiplication rate of parasites (iii) parasite cytoadhesion (iv) multiplicity of infection (v) drug resistance (vi) host defences (vii) host genetic factors [68] (Figures 3a and 3b). Apart from these factors gametocyte carriage among people with asymptomatic malaria is also an important factor to be looked for in order to estimate the reservoirs of infection in malaria-endemic settings.
To achieve successful elimination and finally eradication of VBDs from the world, it is also evident that more work is urgently needed to delineate appropriate strategies to reduce the burden of asymptomatic/ chronic infections across the endemic countries and survey on the presences and the prevalence of asymptomatic cases in diverse disease settings is recommended in order to curb malaria transmission globally.
References
- World Health Organisation (2014) Facts sheet-Vector borne diseases.
- Roshanravan B, KariE, Gilman RH, Cabrera L, Lee E, et al. (2003) Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg 69: 45-52.
- Van Baarlen P, Van Belkum A, Summerbell RC, Crous PW, ThommaBPHJ (2007) Molecular mechanisms of pathogenicity: how do pathogenic microorganisms develop cross-kingdom host jumps? FEMSMicrobiol Rev 31: 239-277.
- World Health Organization (2004) Microbial Factsheets.
- Gubler DJ (2009) Vector-borne diseases. Rev Sci Tech 28: 583-588.
- PatzJA, Githeko AK, McCarty JP, Hussein S, Confalonieri U (2008) Climate change and infectious diseases. Climate Change and infectious Diseases-WHO Chp 6: 103-132.
- Bacaer N, Guernaoui S (2006) The epidemic threshold of vector-borne diseases with seasonality. Journal of Mathematical Biology 53: 421-436.
- VercosaBL, Lemos CM, MendoncaIL, Silva SM, De Carvalho SM, et al. (2008) Transmission potential, skin inflammatory response, and parasitism of symptomatic and asymptomatic dogs with visceral leishmaniasis. BMC Vet Res 4: 45.
- Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K (2013) Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion 53: 2567-2574.
- Pethleart A, Prajakwong S, Suwonkerd W, Corthong B, Webber R, et al. (2004) Infectious reservoir of Plasmodium infection in Mae Hong Son province, north-west Thailand. Malar J 3: 1.
- Riggs MM, Sethi AK, ZabarskyTF, Eckstein EC, Jump RL, et al. (2007) Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 45: 992-998.
- CalderonG, Fernandez R, Valle J (1995) Especies de la fauna anofelina, su distribucion y algunas consideraciones sobre su abundancia e infectividad en el Peru.RevPeruEpidemiol 8: 5-23.
- Dal-Bianco MP, Koster KB, Kombila UD, KunJF, GrobuschMP, et al. (2007) High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg 77: 939-942.
- Eke RA, Chigbu LN, Nwachukwu W (2006) High prevalence of asymptomatic Plasmodium infection in a suburb of Aba Town, Nigeria. Ann Afr Med 5: 42-45.
- Favier C, Schmit D, Müller-Graf CD, Cazelles B, Degallier N, et al. (2005) Influence of spatial heterogeneity on an emerging infectious disease: the case of dengue epidemics. ProcBiolSci 272: 1171-1177.
- Amirshekari MB, Nateghpour M, Raeisi A, Haghi AM, Farivar L, et al. (2016) Determination of asymptomatic malaria among Afghani and Pakistani Immigrants and native population in South of Kerman Province, Iran. Iran J Parasitol 11: 247.
- Mutyaba I, Byakika-Kibwika P, Phipps W, Casper C, Kamya M (2016) Soluble Markers of B-Cell stimulation during asymptomatic and symptomatic malaria parasitemia in children in Uganda. J Global Oncol: JGO004200.
- Owusu ED, Buabeng V, Dadzie S, Brown CA, Grobusch MP, et al. (2016) Characteristics of asymptomatic Plasmodium spp. parasitaemia in Kwahu-Mpraeso, a malaria endemic mountainous district in Ghana, West Africa. Malar J 15: 38.
- Vasquez-JimenezJM, Arevalo-Herrera M, Henao-Giraldo J, Molina-Gomez K, Arce-Plata M, et al. (2016) Consistent prevalence of asymptomatic infections in malaria endemic populations in Colombia over time.Malar J 15: 70.
- Maradiaga A, GarciaJS, Mejia-Torres RE, Escober L, Matamoros J, et al. (2016) Asymptomatic Malaria Infections in an Endemic City of Honduras. Human Parasit Dis 8: 37.
- Balogun ST, Sandabe UK, BdliyaDN, Adedeji WA, Okon KO, et al. (2016) Asymptomatic falciparum malaria and genetic polymorphisms of Pfcrt K76T and Pfmdr1 N86Y among Almajirai in northeast Nigeria. J Infect Dev Count 10: 290-297.
- Degefa T, Zeynudin A, Zemene E, Emana D, Yewhalaw D (2016) High prevalence of gametocyte carriage among individuals with asymptomatic malaria: Implications for sustaining malaria control and elimination efforts in Ethiopia. Human Parasit Dis 8: 17.
- Mvumbi DM, Bobanga TL,Melin P, De Mol P, KayembeJM, et al. (2016) High Prevalence of Plasmodium falciparum infection in asymptomatic individuals from the Democratic Republic of the Congo. Malar Res Treat 2016: 1-4.
- Mogtomo ML, Foko LP, OkoubalimbaEV, Enyegue EE, Ngane AR (2016) High risk of transfusion-transmitted malaria (TTM) from student blood donors living in the town of Douala, Cameroon. J Clin Infect Dis Pract 1: 1-5.
- Bejon P, Williams TN, Liljander A, Noor AM, Wambua J, et al. (2010) Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med 7: e1000304.
- Baum E, Sattabongkot J, Sirichaisinthop J, Kiattibutr K, Jain A, et al. (2016) Common asymptomatic and submicroscopic malaria infections in Western Thailand revealed in longitudinal molecular and serological studies: a challenge to malaria elimination. Malar J 15: 333.
- Dinko B, Oguike MC, LarbiJA, Bousema T, Sutherland CJ (2013) Persistent detection of Plasmodium falciparum, P. malariae, P. ovalecurtisi and P. ovalewallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int J Parasitol Drugs Drug Resist 3: 45-50.
- Bruce MC, Donnelly CA, Packer M, Lagog M, Gibson N, et al. (2000) Age-and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology 12: 247-256.
- Coura JR, Suarez-Mutis M, Ladeia-Andrade S (2006) A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection-a review. Memorias do InstitutoOswaldo Cruz 101: 229-237.
- Al Serouri AW, Grantham-McGregor SM, Greenwood B, Costello A (2000) Impact of asymptomatic malaria parasitaemia on cognitive function and school achievement of school children in the Yemen Republic. Parasitology 121: 337-345.
- Starzengruber P, Fuehrer HP, Ley B, Thriemer K, Swoboda P, et al. (2014) High prevalence of asymptomatic malaria in south-eastern Bangladesh. Malar J 13: 1.
- Alves FP, Durlacher RR, MenezesMJ, Krieger H, Silva LH, et al. (2002) High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg 66: 641-648.
- Gray KA, Dowd S, Bain L, Bobogare A, Wini L, et al. (2013) Population genetics of Plasmodium falciparum and Plasmodium vivax and asymptomatic malaria in Temotu Province, Solomon Islands. Malar J 12: 1.
- De Mast Q, Syafruddin D, Keijmel S, Riekerink TO, Deky O, et al. (2010) Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica 95: 1068-1074.
- Das A, Anvikar AR, CatorLJ, Dhiman RC, Eapen A, et al. (2012) Malaria in India: the center for the study of complex malaria in India. Acta Trop 121: 267-273.
- Sharma J, Dutta P, Khan SA (2016) Epidemiological study of malaria cases in North East region of India. Indian J Med Microbiol 34: 261-262
- Alemu G, Mama M (2016) Assessing ABO/Rh Blood group frequency and association with asymptomatic malaria among blood donors attending Arba Minch blood bank, South Ethiopia. Malar Res Treat 2016: 8043768
- Francine N, Damien B, Anna F, Michael K, ChristevyVJ, et al. (2016) Characterization of asymptomatic Plasmodium falciparum infection and its risk factors in pregnant women from the Republic of Congo. Acta Trop 153: 111-115.
- Doolan DL, Dobano C, Baird JK (2009) Acquired immunity to malaria. ClinMicrobiol Rev 22: 13-36.
- (2011) WHO
- Carrington LB, Simmons CP (2014) Human to mosquito transmission of dengue viruses. Front Immunol 5:290.
- Burke DS, Nisalak A, Johnson DE, Scott RM (1988) A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg38: 172-180.
- EndyTP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, et al. (2002) Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in KamphaengPhet, Thailand. Am J Epidemiol156: 40-51.
- Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, et al. (2006) High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health 11: 935-942.
- Wilder-Smith A, Chen LH, Massad E, Wilson ME (2009) Threat of dengue to blood safety in dengue-endemic countries. Emerg Infect Dis 15: 8-11.
- BaatenGG, SonderGJ, van Gool T, KintJA, Van den Hoek A (2011) Travel-related schistosomiasis, strongyloidiasis, filariasis, and toxocariasis: the risk of infection and the diagnostic relevance of blood eosinophilia. BMC Infect Dis 11: 1.
- NguyenNM, KienDT, Tuan TV, Quyen NT, TranCN, et al. (2013) Host and viral features of human dengue cases shape the population of infected and infectious Aedesaegypti mosquitoes. ProcNatlAcadSci U S A 10: 9072-9077.
- Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, et al. (2015) Asymptomatic humans transmit dengue virus to mosquitoes. Proc Nat AcadSci 112: 14688-14693.
- Murgue B, Roche C, Chungue E, Deparis X (2000) Prospective study of the duration and magnitude of viraemia in children hospitalised during the 1996-1997 dengue-2 outbreak in French Polynesia. J Med Virol 60: 432-438.
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, et al. (2000) Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181: 2-9.
- Vikram K, NagpalBN, Pande V, Srivastava A, Saxena R, et al. (2016) An epidemiological study of dengue in Delhi, India. Acta Trop 153: 21-27.
- Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A (1981) Viraemia in patients with naturally acquired dengue infection. Bulletin of the World Health Organization 59: 623.
- Charrel RN, de Lamballerie X, Raoult D (2007) Chikungunya outbreaks-the globalization of vectorborne diseases. N Engl J Med 356: 769.
- Staikowsky F, Le Roux K, Schuffenecker I, Laurent P, Grivard P, et al. (2008) Retrospective survey of Chikungunya disease in Reunion Island hospital staff. Epidemiol Infect 136: 196-206.
- Staples JE, Fischer M (2014) Chikungunya virus in the Americas—what a vector borne pathogen can do. New Eng J Med 371: 887-889.
- Kumar NCVM, Vishnuwardhan R, Raghavareddy Y, SaigopalDVR (2016) A study on Chikungunya outbreak in Andhra Pradesh, South India. Indian J Med Res 7: 169-181.
- Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M (2007) Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7: 319-327.
- National Institutes of Health (2007) Understanding emerging and re-emerging diseases. Curriculum supplements series.
- Scaria V, Hariharan M, Maiti S, Pillai B, Brahmachari SK (2006) Host-virus interaction: a new role for microRNAs. Retrovirology 3: 1.
- Lederberg J (2000) Infectious history. Science 288: 287-293.
- Alberts B, Johnson A, Lewis J, Raff M (2002) Cell junctions, cell adhesion, and the extracellular matrix. Molecular Biology of the Cell.
- Bright AT, Tewhey R, Abeles S, Chuquiyauri R, Llanos-Cuentas A, et al. (2012) Whole genome sequencing analysis of Plasmodium vivax using whole genome capture. BMC Genomics 13: 262.
- Morse SS (2001) Factors in the emergence of infectious diseases. Emerg Infect Dis 8-26.
- Tyagi S, Pande V, Das A (2014) New insights into the evolutionary history of Plasmodium falciparum from mitochondrial genome sequence analyses of Indian isolates. Mol Ecol 23: 2975-2987.
- Das A (2015) The distinctive features of Indian malaria parasites. Trends Parasitol31: 83-86.
- Badrane H, Tordo N (2001) Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. JVirol75: 8096-8104.
- Archie EA, Luikart G, Ezenwa VO (2009) Infecting epidemiology with genetics: a new frontier in disease ecology. Trends EcolEvol 24: 21-30.
- Galatas B, Bassat Q, Mayor A (2016) Malaria parasites in the asymptomatic: looking for the hay in the haystack. TrendParasitol 32: 296-308.
- Yukich JO, Taylor C, EiseleTP, Reithinger R, Nauhassenay H, et al. (2013) Travel history and malaria infection risk in a low-transmission setting in Ethiopia: a case control study. Malar J 12: 33.
- World Health Organisation (2016)
- Yukich JO, Butts J, Miles M, Berhane Y, Nahusenay H, et al. (2014) A description of malaria sentinel surveillance: a case study in Oromia Regional State, Ethiopia. Malar J 13: 88.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 21104
- [From(publication date):
December-2016 - Aug 23, 2025] - Breakdown by view type
- HTML page views : 19806
- PDF downloads : 1298