Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
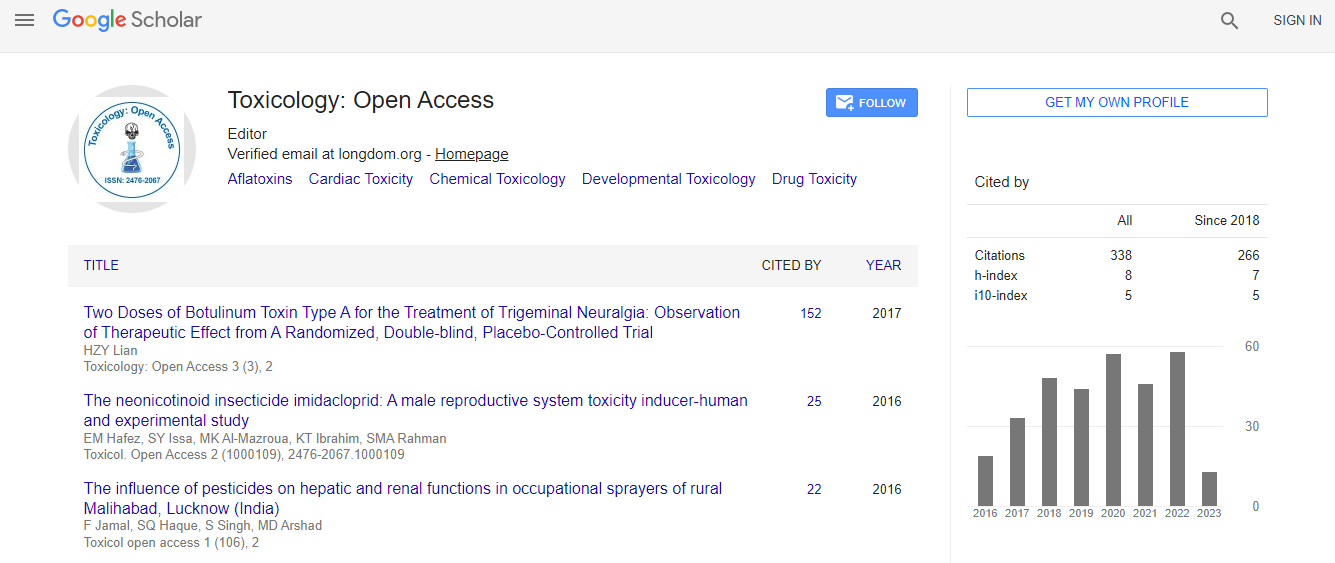
Google Scholar citation report
Citations : 336
Toxicology: Open Access received 336 citations as per Google Scholar report
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Alternatives to animal experimentation
8th World Congress on Toxicology and Pharmacology
Mukul P Pore
Intox Pvt. Ltd., India
ScientificTracks Abstracts: Toxicol Open Access
Abstract
Thousands of new chemicals need to be evaluated every year for safety and efficacy. Millions of animals are used to test safety and effectiveness of a wide range of consumer products including drugs, cosmetics, household products, pesticides, industrial chemicals etc. Because of the widespread use of chemicals in everyday life, we are exposed to variety of natural and man-made chemicals. Every day one new chemical is being added. Use of animals in toxicity testing has increased immensely. These animal tests are time intensive and costly. Also there is a growing public criticism for the use of animals. If we do not experiment on animals, how will we derive our discoveries, our cures? Alternate methods therefore are an absolute necessity. There are various good reasons for development and validation of non-animal alternatives and testing strategies for toxicity testing considering all scientific, economic, logistical, ethical and legal aspects. Last decades, significant efforts have been undertaken to develop alternative methods to assess toxicity. A range of non-animal methods are available. These alternative test methods are developed and validated using Reduction, Replacement and Refinement ��? 3 R��?s approach. Considerable progress in the development of alternative methods have been made in some fields such as ��? dermal toxicity, ocular toxicity, reproductive and developmental toxicity, carcinogenicity, hepatotoxicity, neurotoxicity and biological testing. Of these assays, some are scientifically validated while others are still under development. In this presentation, some important alternative assays will discuss in short. Advantages and limitations of these alternate methods will also be discussed.Biography
Mukul P Pore is one of the founders and is the Lifetime Director of INTOX Pvt. Ltd. which is a well-known GLP certified contract research organization. He is a Diplomate of the American Board of Toxicology (DABT), European Registered Toxicologist (ERT) and Fellow of Indian Society of Toxicology (FST). He has designed and conducted number of toxicology studies for diverse kind of products - pharmaceuticals, agrochemicals, biotechnology products, specialty chemicals, vaccines, medical devices, industrial chemicals etc., during his experience of over 28 years in regulatory/descriptive toxicology. Since 1996, he has played an important role in establishing and bringing INTOX to international standard and repute. He is an Ad Hoc specialist for AAALAC International, USA (2010-2013; 2013-2016; 2016- 2019). He is member of many professional bodies/societies including Indian Society of Toxicology (STOX), Chinese Society of Toxicology, Japanese Society of Toxicology (JST), UK Registry of Toxicology and Laboratory Animal Scientists Association of India. He was nominated on ‘REACH Expert Committee” as “Expert in the field of Environment, Health and Safety” by Ministry of Chemicals & Fertilizers, Govt. of India (2015). He was nominated as Advisor of Editorial Board of “Toxicology International” journal in 2009.
Email: mukulpore3@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi