Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
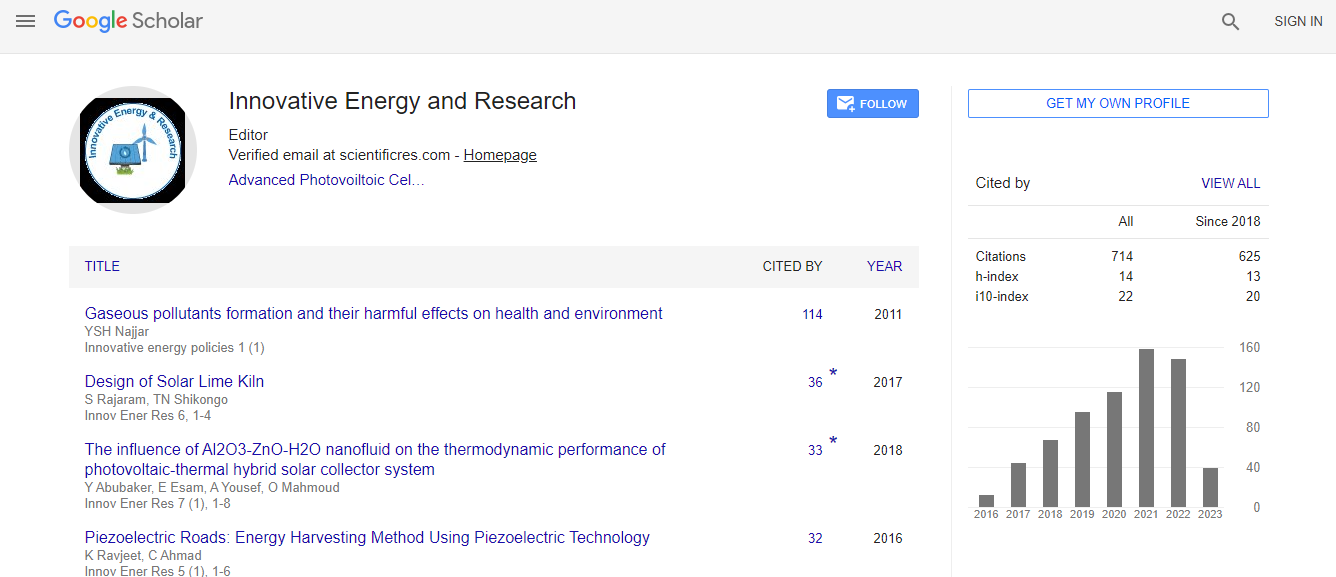
Google Scholar citation report
Citations : 712
Innovative Energy & Research received 712 citations as per Google Scholar report
Innovative Energy & Research peer review process verified at publons
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Combinedoxidation of methane to synthesis gas
20th International Conference on Advanced Energy Materials and Research
Dossumov K, Yergaziyeva G, Myltykbayeva L and Telbayeva M
Al-Farabi Kazakh National University, KazakhstanThe Institute of Combustion Problems, Kazakhstan
Posters & Accepted Abstracts: Innov Ener Res
Abstract
Biogas is a promising source of hydrocarbons capable of providing the current and future needs of mankind for energy and hydrocarbon feedstocks [1]. The main components of biogas obtained in anaerobic bioreactors are methane and carbon dioxide, as impurities can contain carbon monoxide and oxygen [2]. Biogas obtained in this way can be converted to synthesis gas by dry (CH4 + CO2) or steam reforming (CH4 + H2O) using appropriate catalysts [3-5]. In the present work, a modified nickel catalyst supported on alumina was used as catalysts for the combined oxidation of methane. Experiments on testing the efficiency of catalysts were carried out on an automated flow catalytic unit (PKU-1). The reaction products were identified chromatographically on a device of "CHROMOS GC-1000" . The figure shows the results of the effect of promoting additions of lanthanum (La2O3), molybdenum (MoO3) and cerium (CeO2) oxides on the catalytic activity of the nickel catalyst in the combined methane oxidation reaction involving oxygen, carbon dioxide and water vapor, under process conditions: CH4: CO2 : O2: H2O = 2: 2: 1: 0.5, Tr = 850° C and W = 1000 h-1. As can be seen from the figure, the promotion of nickel catalyst with cerium, lanthanum or molybdenum oxides results in an increase in the activity and selectivity of nickel catalyst. The highest activity and selectivity of the nickel catalyst is observed when oxides of cerium and molybdenum are promoted. On the promoted 3% NiO-1% MoO3 / Al2O3 catalyst, the selectivity for hydrogen was 66%, for carbon monoxide 47%. At the same time, the concentration of H2 reaches 52 vol. %, CO up to 33.9 vol. % and methane conversion 95%. For a catalyst of 3% NiO-1% CeO2/Al2O3, the selectivity for hydrogen is 50% and for carbon monoxide 60%. In this case, the concentration of H2 obtained reaches 41 vol.%, CO up to 42.8 vol.% and methane conversion 98%. Thus, the activity and selectivity of oxidesupported nickel-containing catalysts promoted by MoO3, CeO2 and La2O3 oxides during methane reforming into synthesis gas was tested. It is determined that the promotion of nickel catalyst with cerium or molybdenum oxides leads to an increase in the selectivity and activity of the catalysts for the desired products.Biography
E-mail: dossumov50@mail.ru

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi