Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
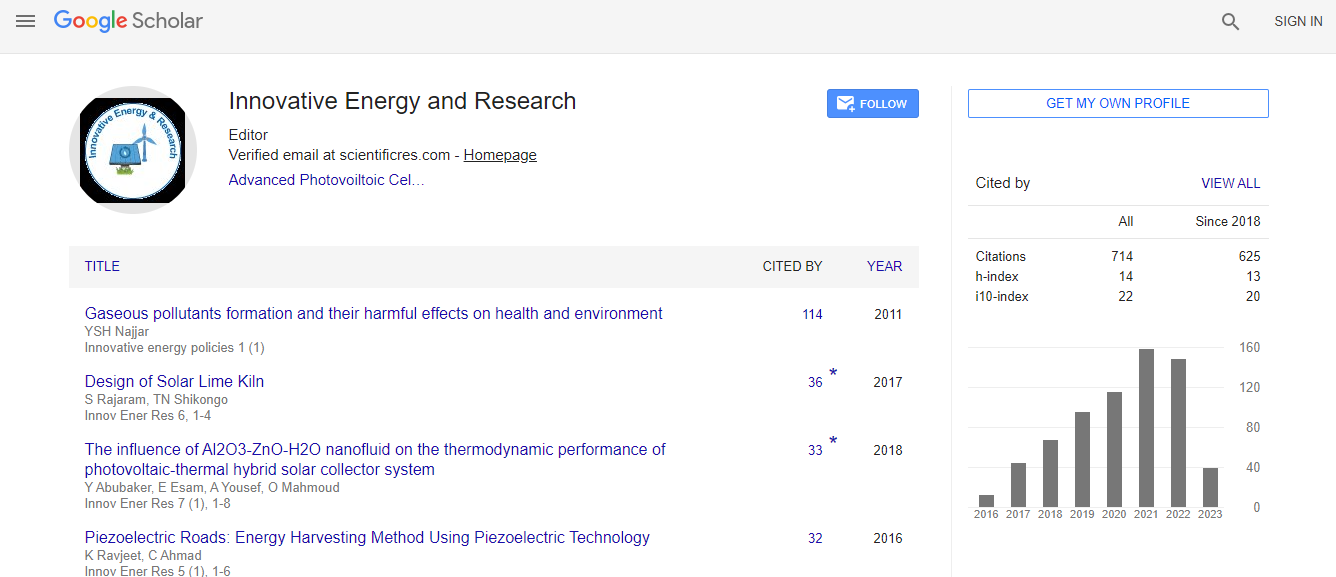
Google Scholar citation report
Citations : 712
Innovative Energy & Research received 712 citations as per Google Scholar report
Innovative Energy & Research peer review process verified at publons
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Materials for thermochemical energy storage: Experimental investigation of cycling stability
20th International Conference on Advanced Energy Materials and Research
M. Gollsch, J. Stengler, M. Spindler and M. Linder
Institute of Engineering Thermodynamics,German Aerospace Center (DLR), Germany
ScientificTracks Abstracts: Innov Ener Res
Abstract
Thermochemical energy storage (TCS) uses the reaction enthalpy of reversible chemical reactions. This storage technology contains a so far largely untouched potential: in comparison to sensible and latent thermal energy storage, TCS offers potentially higher storage densities, the possibility of long-term storage as well as the option to upgrade the thermal energy. This upgrade can be realised if the reaction system consists of a solid and a gaseous component. For these gas-solid reactions with the generic equation the equilibrium temperature is dependent on the reaction gas partial pressure: the higher the partial pressure, the higher the reaction temperature. Consequently, the charging of the storage can take place at lower temperatures than the discharging by adjustment of the reaction gas partial pressure. Currently, a number of water vapour-solid reactions are investigated as thermochemical storage materials [1-4]. Apart from a general suitability of a reaction system for thermochemical storage, special attention has to be paid to the cycling stability of the reaction. This is often done using thermogravimetric analysis [5]. However, past scale-ups have shown that behaviour of bulks differs from that of analysis amounts [6]. The bulk’s changing properties, however, have proven to be crucial for storage reactor design. The investigation of the cycling stability and reaction behaviour of reacting solid bulks has been our motivation to design and build a cycling test bench. In this experimental setup the gaseous reaction partner is water vapour and can be provided at pressures between 5 kPa and 0.5 MPa. Reactor temperatures can be up to 500 °C. The aim of the presented studies is the automated cycling of about 100 ml solid storage material of reaction systems that have previously shown promise at analysis scale. Recent Publications 1. Gutierrez, A., Ushak, S., Linder, M. (2018) High Carnallite-Bearing Material for Thermochemical Energy Storage: Thermophysical Characterization. ACS Sustainable Chemistry and Engineering 6(5):6135-6145 2. Afflerbach, S., Kowald, T., Trettin, R. (2017) Phase transformations during de- and rehydration of scholzite CaZn2(PO4)2•2H2O. Journal of Solid State Chemistry 254:184-194 3. Stengler, J., Ascher, T., Linder, M. (2017) High temperature thermochemical heat transformation based on SrBr2. 12th IEA Heat Pump Conference, 15th-18th May, 2017, Rotterdam 4. Molenda, M., Stengler, J., Linder, M., Wörner, A. (2013) Reversible hydration behavior of CaCl2 at high H2O partial pressures for thermochemical energy storage. Thermochimica Acta 260:76-81 5. Richter, M., Habermann, E.-M., Siebecke, E., Linder, M. (2018) A systematic screening of salt hydrates as materials for a thermochemical heat transformer. Thermochimica Acta 659:136-150 6. Schaube, F., Kohzer, A., Schütz, J, Wörner, A., Müller-Steinhagen, H. (2013) De- and rehydration of Ca(OH)2 in a reactor with direct heat transfer for thermos-chemical heat storage. Part A: Experimental results. Chemical Engineering Research and Design 91:856-864.Biography
Marie Gollsch has a degree in environmental engineering and has been at the German Aerospace Center since 2012. She is part of the research area “Thermochemical Systems” at the department of Thermal Process Technology within the Institute of Engineering Thermodynamics. She specialises in the investigation and evaluation of thermophysical properties of thermochemical storage materials with focus on gas-solid reaction systems with water vapour as gaseous component. Additionally, she has expertise in the study of structural changes of the solid components of gas-solid reaction systems which occur due to the cycling of the storage material.
E-mail: Marie.Gollsch@dlr.de

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi