Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
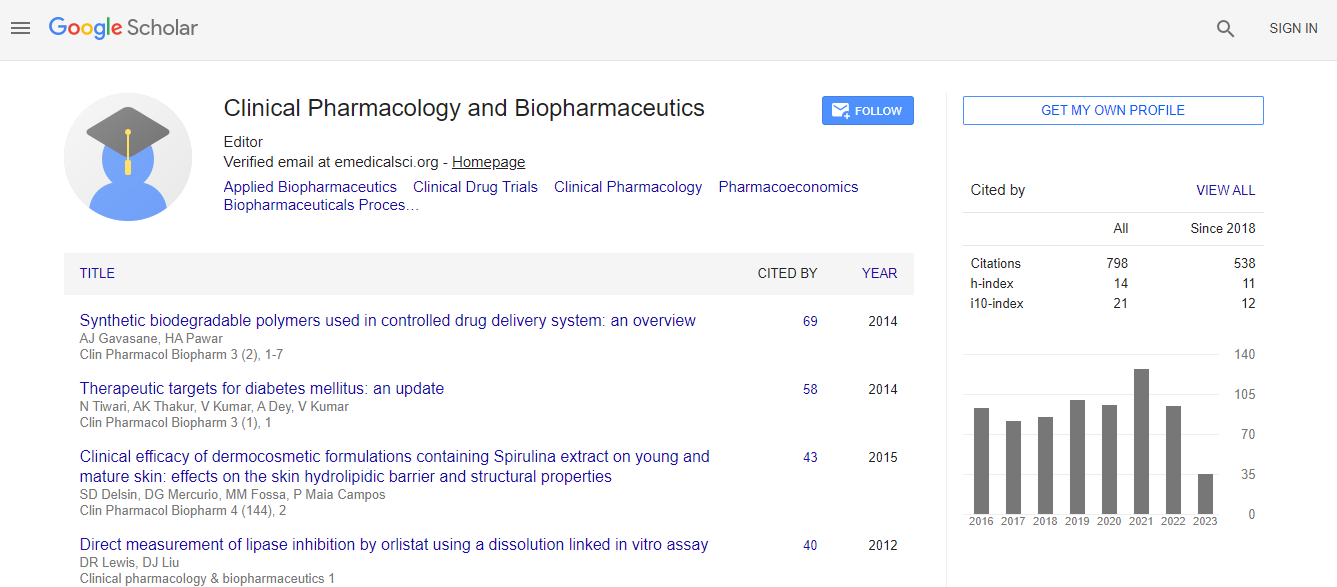
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Tamper-resistant drug formulations - How effective they really are?
International Conference and Expo on Biopharmaceutics
Alex Nivorozhkin
Neo-Advent Technologies, USA
ScientificTracks Abstracts: Clin Pharmacol Biopharm
Abstract
Abuse of opioids and other prescription controlled substances is a recognized nation-wide problem in United States and around the globe. Emerging FDA guidelines are setting up the ground rules for related evaluation and product labeling. As these documents point out, science of abuse deterrence is relatively new, and both the formulation technologies and the analytical, clinical, and statistical methods for evaluating those technologies are rapidly evolving. This presentation will focus on the overview of the existing and novel formulation approaches to enable tamper-resistant properties to include approaches involving physical/chemical barriers, agonist/antagonist combinations, aversion, delivery, and prodrug. Neither of the approaches can provide absolute protection and eliminate all the illicit options. So the question is, if there are clear pathways is assessing suitability of an appropriate technology for a given drug candidate or product and evaluating their readiness to be deployed. Up to date only a handful of tamper-resistant technological solution advanced far enough to be implemented into the market product. This number will certainly increase in near future as nascent and established tamper-resistant formulations will be further tested in clinical trials and interrogated by FDA.Biography
Alex Nivorozhkin is an Entrepreneur and a Team builder in life sciences’ arena with vast experience and track record in an early technology transfer and development. He was a Co-founding Member of Boston BioCom, LLC, a biopharma company funded by the seed investment from Pfizer, Neo-Advent Technologies LLC, Amorsa Therapeutics, an emerging company developing new medications and formulation approaches for treatment of the refractory pain, and other ventures. He gained substantial experience in the commercial aspects of drug discovery and development at Epix Medical and Inotek Pharmaceuticals where he served as the company’s Head of Medicinal Chemistry. He was as a Senior Program Manager at the Center of Integration of Medicine and Innovative Technologies (CIMIT) at Massachusetts General Hospital, Draper Laboratory and MIT aimed at developing new medical devices; and a Scientific Programs Officer at Sheldon and Miriam Adelson Medical Research Foundation. He is a Co-inventor of several drug candidates that have advanced to clinical trials and late pre-clinical studies in the United States, has co-authored over 60 scientific publications in different areas of chemistry, chemical biology, and material sciences and holds more than 20 patents. He received a PhD in Physical Organic Chemistry from Rostov University and conducted the Postdoctoral research at the University Paris-Sud, France, and the Department of Chemistry and Chemical Biology, Harvard University. He is a Member of the American Chemical Society, Controlled Release Society, American Association of the Pharmaceutical Sciences and other professional organizations, active on the conference circle and serves as a co-chair of the Formulation and Drug Delivery Committee of the Massachusetts Biotechnology Council.
Email: aln@neo-adventtec.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi