Effect of Flavanoidal Rich Novel Vesicular Cream on the Cellular Components of Skin Against UV Irradiation
Received: 03-Oct-2017 / Accepted Date: 12-Feb-2018 / Published Date: 21-Feb-2018 DOI: 10.4172/2476-2253.1000113
Abstract
Skin cancer is a progressive disease which causes degradation of ECM components that leads to inflammation of skin, formation of wrinkles, epidermal hyperplasia and disturbances in the dermal collagen and elastin bundles. Exposure of UV radiation causes release of various enzymes and chemicals mediators which acts as a marker in the detection of cellular degeneration. MMP-1 and TNF-α are markers of cell damage by UV irradiation. Since skin cancer hampers the normal cellular functions, the property of the skin to rejuvenate and replenish itself gets destroyed. We, in our study have put forward an approach to treat the UV radiation caused skin cancer naturally by formulating novel vesicular cream of Aloe vera. The novel vesicle selected was ethosomes because of their good skin penetration property. Aloe vera is used as the phytosources because of its proven ability in treating skin complications. Through our study we have tried to prove the successful encapsulating efficacy of ethosomes particularly for herbal extracts and its skin targeting potentials. Aloe vera extract incorporated ethosomes were developed as cream formulations whose efficacy was established through different skin parameters in vitro and in vivo.
Keywords: Ethosomes; Extracellular Matrix; Aloe vera; Collagen; Herbal formulation; Biological oxidative damage
Introduction
Skin plays a crucial role in defence mechanism against various environmental and pathological factors. It is the major site of irradiation of ultraviolet radiations, pollution, dust and dirt. The sign and symptoms of pathological onset of some diseases arise initially on skin surface. Therefore, it is very important to preserve the vitality of the skin [1-5].There is an ardent need to develop herbal topical formulations which could be effective and mild. The current research scenario relies on the concept of designing novel cargoes for efficient delivery of phyto-constituents [6-9]. It has not only rationalised the therapeutic efficacy of drugs, but also limited their toxicity ratio, enhanced bioavailability with improved pharmacokinetic profile [10]. Ultraviolet radiation plays a crucial role in the degradation of Extracellular Matrix components (ECM) leading to collagen damage, wrinkle formation and several other skin complications in younger as well as aged skin. This study potentiates that herbal formulation could be used as a potential skin rejuvenating agent and with remarkable defending against the harm caused due to solar radiations. Aloe vera is a very known plant being employed in cosmetics for a longer time and exert significant radical scavenging effect. Such herbs when incorporated into a suitable vesicular form i.e. ethosomes, exhibit a number of therapeutic actions at cellular level as well as at the Extracellular matrix (ECM) level. Therefore, our idea behind this study was to develop a novel vesicular system of Aloe vera and inoculate it into a suitable formulation and characterizes its ability to prevent ultra violet radiation induced skin damage for prolonging youthful skin [10].
Materials
Aloe vera fresh leaves were collected from the medicinal garden of the University Institute of Pharmacy, located in Chhattisgarh State, India. And the sample was authenticated with the help of the herbarium of the Pharmacognosy department of University Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur, (C.G.) India. All other reagents used were of analytical grade. The cell lines were obtained from the National Centre for Cell Science, Pune, India. All the human and animal studies were conducted as per their ethical guidelines taking permission from the Institutional Ethical Committee.
Experimental Methods
Herbal extract loaded ethosomes was prepared by taking hydroethanolic extract of A. vera at a ratio of 10:9 with slight modification of the cold methodas described by Dayan and Touitou in 2000 [11-13]. Initially (1-3%) of soya phosphatidylcholine was taken and dissolved in (10-40%) of 90% ethanol by use of magnetic stirrer (Remi Motors Mumbai) in a completely closed flask at 30ºC. To this solution 10 mg hydro alcoholic extract dissolved in hot distilled water (having temperature 30ºC) (to make the volume up to 100%) was added as a fine stream by the use of syringe very slowly and then whole system was stirred for 15 min at 900 rpm. Further they were sonicated for 5-15 min. Finally, the formulations were stored under refrigeration [11]. The optimization was done on the basis of vesicular size, polydispersity index and percent entrapment efficiency [14,15]. After optimization of ratio of components, the herbal extract loaded ethosomes was prepared to take optimized ratio of extract individually and further evaluation parameters were performed.
Preparation of photo-protective cream formulations
Preparation of base cream: Base cream (BC) was prepared by using a phase inversion technique [16]. The internal phase was prepared in the vessels in which the emulsification was carried out according to the composition depicted in Table 1. Initially oily constituents, cetyl alcohol, stearic acid, olive oil, jojoba oil, tea tree oil, polysorbitan mono-oleate, polysorbitan stearate, were mixed using magnetic stirrer at 200 ± 25 rpm at 65-75° C. After the complete melting a 50 ml portion of deionised water (70 ± 2°C) was added at a rate of 30 ml min-1 at increased speed 275 ± 25 rpm. When the temperature of the internal phase was reduced to 50ºC, phase inversion took place and the solution became viscous, the remaining aqueous phase containing propylene glycol and glycerine was then added. When the temperature was reduced to 40ºC, lemon grass oil and lavender oil were added to the mixture and further by continuous stirring semisolid cream base was obtained [5,17,18].
| Name of Excipients | Quantity (w/w% ) |
|---|---|
| Oily constituents Cetyl alcohol Stearic acid Olive oil Jojoba oil Tea tree oil Lemon grass oil Lavender oil Polysorbitan monostearate (Tween 80) Polysorbitan monooleate (Span 60) Aqueous constituents Glycerin Propylene glycol Deionised water q.s. (100 gm.) |
3.56 4.80 5.78 0.50 0.51 0.50 0.25 0.75 1.78 3.53 4.02 q.s. |
Table 1: Composition of base cream.
Preparation of herbal extract loaded conventional cream formulations: Herbal extract loaded conventional creams were prepared by the phase inversion technique as the base cream was prepared [19]. All the oily constituents were mixed at 65-75°C at 200 ± 25 rpm [20]. After the complete melting along with the deionised water the herbal extract (1.0%, 1.5% and 2%) was added to produce the creams (AC1, AC2 and AC3)
Preparation of novel cream formulations without extract: The novel vesicular creams were prepared by incorporating the empty novel vesicular systems in the cream. The procedure and constituents of the cream preparation were same as the base cream [20-22]. While preparing novel creams, the optimization was done by replacing 15%, 20% and 25% of deionised water by the novel vesicular formulations. Care was taken that the vesicular systems were added when the temperature was reduced to 30°C so that they could withstand the temperature. The optimized concentration of nano-vesicular dispersion addition was selected on the basis of physicochemical parameters of creams namely pH, consistency, stability. Incorporation of 20% nano-vesicular dispersion into the cream produced the best results, hence this percentage was the optimized percentage for the preparation of ethosomes incorporated cream (EC0) [23].
Preparation of herbal extract loaded novel cream formulations: The novel herbal extract loaded vesicular creams (AEC1, AEC2 and AEC3) were prepared by incorporating the herbal extract loaded novel vesicular systems in the cream. The procedure and constituents of the cream preparation were same as the base cream. While preparing the novel creams, the optimization was done by replacing 15%, 20% and 25% of the aqueous phase by herbal extract loaded novel vesicular formulation, ethosomes in case of ethosomal creams . The vesicular creams prepared by incorporation of 15%, 20% and 25% vesicular dispersion was observed and the creams with 20% vesicular dispersion was found stable. In other two types the creams rheological properties were disturbed. Hence for the preparation of cream formulations the 20% of deionized water was replaced with the prepared nanovesicular systems.
In vitro evaluation of novel vesicular formulations
Vesicular size, polydispersity index and zeta potential determination: The z-average diameters of vesicles were determined by Dynamic light scattering (DLS) method using a Malvern Zetasizer 4700 (Malvern Ltd., Malvern, UK) with a 25 mW He-Ne laser and the Automeasure (version 3.2) software. The samples were diluted in order to avoid multiple scattering. As a measure of the particle size distribution, the polydispersity index was calculated. The measurements were performed at 27°C at an angle of 90° between laser and detector. For each vesicle composition, four suspensions were measured in triplicate by DLS [9,19].
Vesicular shape and morphology: The morphological characterization of optimized vesicle was done using a digital Labomed microscope (Leica ATC 2000) with the camera at 10-40 x resolutions. Formulations were visualized by transmission electron microscopy (TEM) (Morgagni 268-D) at an accelerating voltage of 100 kV [20,21]. A drop of the sample was placed on a carbon-coated copper grid to form a thin film and negatively stained by adding a drop of 1% w/v phosphotungstic acid [19,20]. The grid was allowed to air dry and the samples were viewed and photographed. TEM was performed for ethosomes alone as well as after incorporation into creams to confirm the presence of vesicles after the preparation of cream too.
Entrapment efficiency determination: The entrapment efficiency of the vesicles (ethosomes) was measured by the ultracentrifugation method. Entrapment efficiency was determined by the first separation of the un-entrapped extract by the use of mini-column centrifugation method. Later the prepared vesicles were centrifuged in centrifugation tube and centrifuged at 14,000 rpm for 30 min. After centrifugation, the vesicles were disrupted using 0.1% Triton X-100 [6,7]. The calculation of the entrapment efficiency of the extract was achieved by measuring the aloin for A. vera extract which is the major constituent of A. vera extract. The above stated contents were assayed both in the sediment and in the supernatant. The entrapment efficiency was calculated from the relationship [(T-C)]/T × 100 where T is the total amount of important constituent that is detected both in the supernatant and sediment, and C is the amount of important constituent detected only in the supernatant. The entrapped important constituent of the herbal extract was determined by High performance liquid chromatography.
Experiments were performed on an HPLC system Simadzu-10ATVP, binary gradient equipped with detector Shimadzu UV-VIS SPD-10 AVP, software Spinchrom, Chennai. The separations were performed on column Merck’s {Lichrospher 100, C-18 (250 × 4.6 mm) and ODS RP-18 (250 × 4.6 mm, 5μ particle size)}. The mobile phase used was acetonitrile-water (30:70) at a flow rate 1.0 mLmin-1. The standard curve of aloin was prepared in the concentrations 2-12μg/ml and the area under the curve was determined at 355 nm wavelength. Similarly the appropriate aliquot of ethanolic solution of A. vera extract was prepared and 20μl of it was injected and aloin content was determined by the standard curve of pure aloin [24].
Cell cultures experiment
HaCaT cells: The cell cultures were grown as per manufacturer’s instruction. In brief, 1 × 105 cells were suspended in 20 mL of Dulbecco’s Modified Eagle Medium (DMEM) containing 10% foetal bovine serum (FBS) in a 96 wall plate and incubated at 37ºC with 5% CO2. The cells were harvested on a confluency limit of 90% and seeded in a 6 well plate supplied with 0.05% EDTA and 0.1% trypsin in phosphate buffered saline (PBS) at a density of 5 × 103cells/cm2. The well plates were again incubated with DMEM until 90% confluency was reached prior to treatment [25].
Novel vesicular cream treatment on HaCaT cell cultures: For dosing with novel vesicular systems, each well containing HaCaT cells at 90% confluency was washed with PBS, and 2 mL DMEM with 10% FBS was added. To the media was added 0.5μL, 1.0μL, 2.0μL or 4.0μL of 75mM Novel vesicular systems in DMSO to obtain concentrations of 25μM, 50μM, 100μM and 200μM respectively [26]. The control for both the groups contained 4μL DMSO and vehicle control had neither DMSO nor Novel vesicular systems added to the media. After dosing the cells were again incubated at 37° C with 5% CO2 for 24 h. All doses were done in triplicate.
UVR exposure on HaCaT cell cultures: For UVR exposure cells were removed from the incubator after 24 h and the media removed and replaced with PBS. Samples were then placed under lamps without covers. Cells were exposed for 30 min using a UV lamp (310-365nm) kept at a distance of 20 cm with an intensity of 500-700μW/cm2. PBS was removed after each exposure and fresh 2 mL of DMEM with 10% FBS and samples were again added to each well and again kept under incubation for 24 h. After 24 h, the cell cultures were removed and the media is collected for analysis and stored at a temperature of -80°C freezer for further analysis [27,28].
TNF-α and MMP-Estimation: The exposure of UVR results in the formation of Tumour necrosis factor-α in HaCaT cell lines. In our study we estimated the release of TNF-α, on addition of novel vesicles of A. vera using TNF-α Human ELISA Kit® (Invitrogen, India) and Human MMP-1 ELISA Kit® (RAB0361 SIGMA, India), as per manufacturer instructions. Briefly, the cell cultures were seeded in a 96 well plate supplied with 50μL of culture media and 200 μL of diluent solution and incubated at room temperature for 120 min [28,29]. After that, the samples were aspirated, and the plate was washed thrice and 200 μL of both the conjugates were added to each well and the plates were incubated at room temperature for another 60 min. Followed by this, the novel vesicular solution was removed, plates were washed thrice and again 200 μL substrate solution were added to each well and left incubated in dark for 30 min. To each well 50 μL of stop solution was added and the absorbances were measured at 450 nm and 570 nm using a ELISA plate reader. An eight point calibration curve was prepared using standard provided by the supplier and the TNF-α concentration of each well was determined by interpolation. All the measurements are done in triplicate [30,31].
In vitro evaluation of cream formulation
Various types of cream formulations were developed and the morphology of the vesicles present in the cream formulations were observed by transmission electron microscopy, then all the physicochemical and psychometric parameters were evaluated to check the safety of the product and along with it in vitro .
Morphology of the novel vesicles present in cream: The morphological characterization of novel vesicles incorporated into the cream was done using transmission electron microscopy (TEM) (Morgagni 268-D) at an accelerating voltage of 100 kV. The cream sample was diluted with distilled water and a drop of the sample was placed on a carbon-coated copper grid to form a thin film and negatively stained by adding a drop of 1% w/v phosphotungstic acid. The grid was allowed to air dry and the samples were viewed and photographed.
Physicochemical evaluations of creams as per BIS guidelines: Several physicochemical parameters were measured for each prepared cream formulation according to the Indian Standard Bureau methods. These physicochemical parameters provided information regarding formula stability and skin compatibility. The pH, thermal stability, fatty content and non-volatile content of the prepared formulations were determined according to the Indian standard guideline (IS: 6608-1978B-1, IS: 6608-1978B-2, IS: 6608-1978B-3). Ash examination, saponification values and acid values were determined according to methods discussed by [25]. The viscosity was measured using a Brookfield viscometer (at 3 rpm.) and the layer thickness was evaluated according to Multimer, 1956. Spreadability refers to the % area covered by a fixed amount of cream sample after the uniform spread of sample and layer thickness refers to the thickness of the layer (in microns). All evaluations were carried out in triplicate presented as mean ± standard deviation [SD]. All parameters were statistically analysed at 99% confidence level in the column. Analysis of variance ANOVA and Student’s paired t-tests were performed. Differences were considered statistically significant if p<0.01.
Statistics: Statistical analyses were performed using GraphPad Prism 5.0 (GrpahPad Software). Data was analyzed by one-way ANOVA with a Bonferroni's Multiple Comparison Test. The level of significance was set at P<0.05.
Ex-vivo skin permeation studies
Preparation of a Goat skin: Fresh Goat skin was collected from a local slaughter house. After thorough washing, the hair on the skin was removed using a surgical razor and the skin was separated from the underlying cartilage and subcutaneous fat using a scalpel and cut into appropriate sizes.
Ex-vivo permeation studies: For Ex-vivo permeation study fullthickness goat skin was used for locally fabricated Franz diffusion cell. The skin was clamped between the donor and the receptor chamber of diffusion cell with an effective diffusion area of 2.5 cm2. The receptor chamber was filled with freshly prepared phosphate buffer pH 5.5. The diffusion cell was maintained at 32°C and the solution of the receptor chamber was stirred continuously at 350 rpm by using magnetic stirrer with hot plate (Remi, Mumbai, India). The formulation (4.0 g) was gently placed in the donor chamber. At 1, 2, 3, 4, 5, 6, 12, 18 and 24 h, 5.0 ml of the solution in the receptor compartment was removed and analysed using UV-spectrophotometer (Systronic, UV-Double beam spectrophotometer) and replaced immediately with an equal volume of fresh buffer. All experiments were performed in triplicate.
Biological studies
Photo aging leads to marked cutaneous alterations characterized clinically by deep wrinkles, roughness, sallowness, mottled depigmentation, telangiectasia and a variety of benign and malignant neoplasms. The deleterious effects of ultraviolet radiations not only affect the various biochemical components of skin like skin enzymes, ascorbic acid and proteins but also show marked changes in the histological structure of skin [32-34]. The level of the enzymes which convert reactive oxygen species (ROS) to harmless water and molecular oxygen and thereby protect the skin from ROS-induced damages is altered by the repeated exposure to the ultraviolet radiations. As reactive oxygen species generated by ultraviolet radiations damages the anti-oxidative system of skin and the use of antioxidant phyto-constituents in topical formulations is a very promising approach. Therefore, the biological studies are of marked importance to assess the extent of the effect of ultraviolet radiations and the photo-protective effect of the prepared formulations.
Animal grouping
Animals were divided into eleven groups consisting of six animals in each group. A demarcated area of approximately 2 cm2 on the dorsal surface of the mice was shaved using a soft hair removing cream. A hair removing cream was preferred over a shaving blade to minimize free radical production due to trauma from the blade. To decrease the number of animal groups Site 1, 2 and 3 was taken and the formulations were applied. The formulation was applied 1 h before UV exposure, once daily to the animals of respective groups. The study pattern for the biological studies for each herbal cream formulation was designed according to the following manner as described in Table 2.
| Animals used | Albino rats (Wistar Strain) having body weight 180 ± 20 g |
| Conditions maintained | Controlled Temperature (25 ± 2°C), Relative Humidity (60 ± 5%) |
| Number of animals | 6 animals in each group Group 1: Control (without UV and/or formulation treated) Group 2: UV treated Group 3: Site 1: UV treated plus base cream Site 2: UV treated plus 2.0% A. vera extract loaded cream Group 4: Site 1: UV treated plus empty ethosomal cream Site 2: UV treated plus 2% A. vera extract loaded ethosomal cream |
| Application dose | Single application 2.5 mg/cm2 cream formulation |
| Irradiation | Ultraviolet range lamps ( 200-400 nm range) Irradiance of 3.6 ± 0.4 mW cm-2 to achieve 5 Jcm-2 |
| For Biochemical studies | Wash, clean and remove skin tissues. Weigh skin tissues; mince on glass plate over ice bags to make colloid and homogenate. Centrifuge the homogenate at 5000 rpm for 10 min. Collect the supernatant, store in deep freeze and perform biochemical studies. |
| For Histological Studies | Fix the skin tissues in 10% chilled neutral formalin for 12 h at 4ºC. Rinse with distilled water, dehydrated alcohol in series, cleared in xylene and embed in paraffin wax to make tissue blocks. Cut 8 µm thick series section using rotary microtome. Stain the sections with haematoxylin and eosin stains and take the photomicrographs. |
Table 2: Protocol for biological studies.
Detail of cream formulations with their formulation codes
After physicochemical, psychometric and bioengineering evaluations only 2% extract loaded creams were selected for biological studies and the comparison was done with base cream (BC), empty ethosome incorporated cream (EC0), 2% A. vera extract loaded cream (AC3) and 2% A. vera extract loaded ethosome incorporated cream (AEC3) [32].
Ultraviolet (UV) exposure to animals
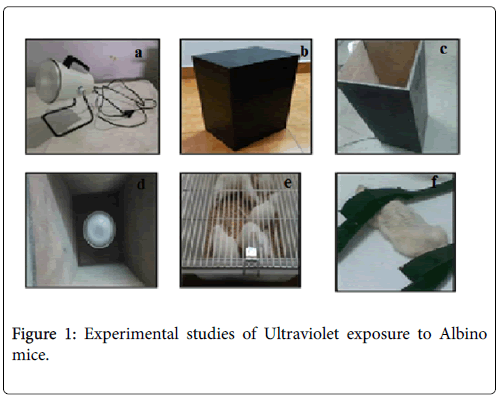
Animal housing: Albino mice (male/female) (Wistar strain) of body weight 180 ± 20 g were used for the present study. The animals were kept in well-ventilated area of the period of experiment till thirty days. The animals were housed in polypropylene cages at standard animal house conditions, temperature (25 ± 2°C), relative humidity (60 ± 5%) and 12 h dark/light cycle. Animals were fed with standard laboratory diet and drinking water ad libitum throughout the experiment. The animals were maintained as per CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) regulations and the experiment was studies were performed after due permission by IAEC (Institutional Animal Ethics Committee) of the Pinnacle Biomedical Research Institute, Bhopal (CPCSEA Reg No. 1283/c/09/ CPCSEA, Ref No. PBRI/IAEC/11/PN-97). Prior to irradiation, mice were anaesthetized using chloroform (few drops) so that the movement of animals would be restricted during the exposure and the irradiation would be homogeneous. In the present experiment an ultraviolet (UV) lamp (200-400 nm) was used to induce photo stress on the skin of mice (Figure 1a). Wooden square chamber having narrow spaces (for breathing air) was fabricated to fit the ultraviolet lamp.(Figures 1b and 1c). The distance from the lamp to the animals’ back was kept constant at 35 cm. At the start of the experiment, the dorsal skin surface of the mice was shaven and it was resting on a wooden surface with arms and legs loosely tied to restrict the movement (Figures 1d-1f). The irradiance of 3.6 ± 0.4 m W/cm2 was exposed to achieve 5 J/cm2 intensity of exposed light radiation. The time of light exposure was calculated according to OECD guidelines by following formula [5].

Biochemical estimations
After 30 days of study, animals were sacrificed under light ether anaesthesia and their skins were surgically removed. The excess of fascia and adherent tissues were removed by washing with the chilled normal saline solution. Portion of skin tissues was weighed and minced on glass plate over ice bags and homogenized to make colloid. Tissue homogenate (10% w/v) were prepared in 0.15 M Tris hydrochloride and centrifuged at 15000 rpm for 15 min and supernatant fractions were collected and stored in deep freeze for further estimation of biochemical parameters. To study the UV protecting effects of different formulations, biochemical parameters were estimated by reported procedures [35].
Superoxide dismutase (SOD) estimation: 0.1 ml of the supernatant fraction of tissue homogenate was taken as a sample and added 1.2 ml sodium pyrophosphate buffer (pH 8.3, 0.052 M), 0.1 ml phenazinemethosulphate (186 μM), 0.3 ml of 300 μM Nitrobutetrazoliumand 0.2 ml NADH (750 μM). Incubated the mixture at 30°C for 90 s. Added 0.1 ml glacial acetic acid, Stirred with 4.0 ml n-butanol. Allowed to stand for 10 min and centrifuged and separated the butanol layer [36]. The absorbance was taken at 560 nm taking butanol as blank. One unit of SOD is the amount of enzyme that inhibits the rate of reaction by 50%.
Interpretation will be based on % inhibition is to SOD concentration curve:
% Inhibition 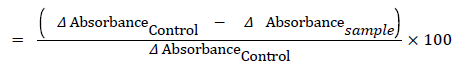
Malondialdehyde estimation: The extent of lipid peroxidation in the mouse skin upon UV exposure was determined in terms of Nano moles of malondialdehyde per mg protein, as discussed by Ohkawa et al. [37]. Malondialdehyde (MDA), an indirect index of lipid peroxidation, was assayed in the form of thiobarbituric acid reacting substances (TBARS).To 0.2 mL of homogenate, 0.2 mL of 8.1% sodium lauryl sulphate, 1.5 mL of 20% acetic acid (pH 3.5), 1.5 mL of 0.8% thiobarbituric acid (freshly prepared), and 0.6 mL of distilled water were added. The mixture was heated for 1 h on a boiling water bath (95°C) for 60 min using glass ball as condenser and then cooled under running tap water. Then 1 mL of distilled water and 5 mL of n-butanol: pyridine (15:1) mixture was added to the mixture. After vigorous shaking for 2 min, the solutions were centrifuged at 3000 rpm (10 min) and the organic layer was separated. The absorbance of organic layer was measured at 532 nm, taking blank as n-butanol: pyridine (15:1) mixture. Results were expressed in equivalent to MDA.
Estimation of ascorbic acid: To 0.2 ml of skin tissue homogenate 0.8 ml of 10% trichloroacetic acid was added, mixed and centrifuged at 1500 rpm. To 0.5 ml of aliquot 0.2 ml of the dinitrophenyl hydrazine solution was added in a tightly closed stoppered flask and incubated at 30ºC for 3 h. After 3 h it was chilled in ice bath and 0.8 ml of cold 65% sulphuric acid was added. After 30 min the absorbance was measured at 530 nm wavelength by UV-Visible spectrophotometer. [5]
Estimation of catalase activity: Catalase activity was determined by slight modification of reported methods. 0.1 ml of the supernatant of tissue homogenate was taken and added 1.9 ml (50 mM phosphate buffer) pH 7.0 and to it added 1.0 ml of freshly prepared 30 mM H2O2. The change in absorbance was measured at 240 nm for 2 min at 30-s intervals. One unit of Catalase will decompose 1.0 micromole of H2O2 per min at pH 7.0 at 25°C). Calculations were done using the following formula:
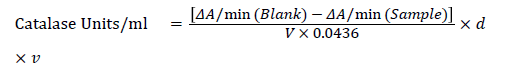
Calculation using Beer’s Law: [H2O2] mM = A240/0.0436
Where,
d=dilution of original sample for catalase reaction
V=sample volume in catalase reaction
0.0436=ε mM for H2O2
V=reaction volume in ml
Estimation of total protein: The protein content of skin homogenates was measured using the Biuret method and bovine serum albumin as the reference protein. In all, 0.1 mL of skin homogenate, 2.9 mL of sodium chloride, and 3 mL of working biuret reagent were mixed together. Biuret reagent was prepared by mixing a solution of sodium potassium tartrate in 0.2 N sodium hydroxide with copper sulphate. Potassium iodide was added to this solution, and the total volume was made up to 100 mL with 0.2 N NaOH. This mixture was kept at room temperature for 10 min, and its absorbance was measured at 540 nm.
Histological studies
For histological studies, skin tissues were fixed in freshly prepared 10% chilled formalin for 24 h at 4°C. After fixation, the skin tissue was rinsed in distilled water several times and dehydrated in graded alcohol series, cleared in xylene and embedded in paraffin wax to make tissue blocks. The 8 μm thick sections were cut by using a rotary microtome. Sections were stained with haematoxylin and eosin stain for demonstration of ultra-structural epidermal and dermal changes.
Human subjects and skin punch biopsies
The human study experiments were performed in accordance with the approved institutional protocol (Human ethical committee reference No.). All volunteers participated in the study gave written informed consent, which was approved by the Institutional Review Board of University Institute of Pharmacy, Pt. Ravi shankar Shukla University , Raipur, India. Six human subjects, both male and female, ranging from 25 to 55 years old, were recruited for this study. Only those individuals were selected which were in good health and having no sign of acute or chronic diseases and having no photo-allergic tendency. Lactating Mothers and Pregnant women were excluded. Firstly their MED was taken. The procedure to carry out this study with slight modifications. MED was determined by taking six sites in sequence and exposing them to UV radiation with gradual increase in incident time from 30 s. After 24 h whichever site expresses redness at a definite UV irradiation dose was considered as MED. Thereafter, Base line readings were taken and thighs skin sites were exposed for irradiation with or without pre-treatment with A. vera ethosomal cream. Volunteers were asked to visit the next day for evaluation of erythema and skin biopsies. Skin Biopsies were taken from each site from every volunteer and snap frozen immediately and stored at −80°C for further use.
Results
The vesicular system selected was ethosomes due to their lipoid nature and efficient delivery of drug as well as phyto-constituents to the different layers of skin. The hydroethanolic extract of Aloe vera gel was prepared and its photo protective ability was assessed on the basis of phytochemical analysis, antioxidant activity, total poly phenolic content and in vitro sun protection factor determination. The ultraviolet radiations not only affect the morphological characteristics but also the structures of deeper dermal layer hence the aim of the work was to prepare stable herbal extract loaded novel vesicles and develop topical creams with the incorporation of these loaded vesicles.
The herbal extract loaded vesicles (ethosomes) was prepared and their various components were assessed on the basis of vesicular size, polydispersity index and percent entrapment efficiency. The determination of entrapment efficiency of the herbal extract was achieved by measuring the important active constituent (Aloin) in A. vera extract.
Entrapment efficiency determination of novel vesicles
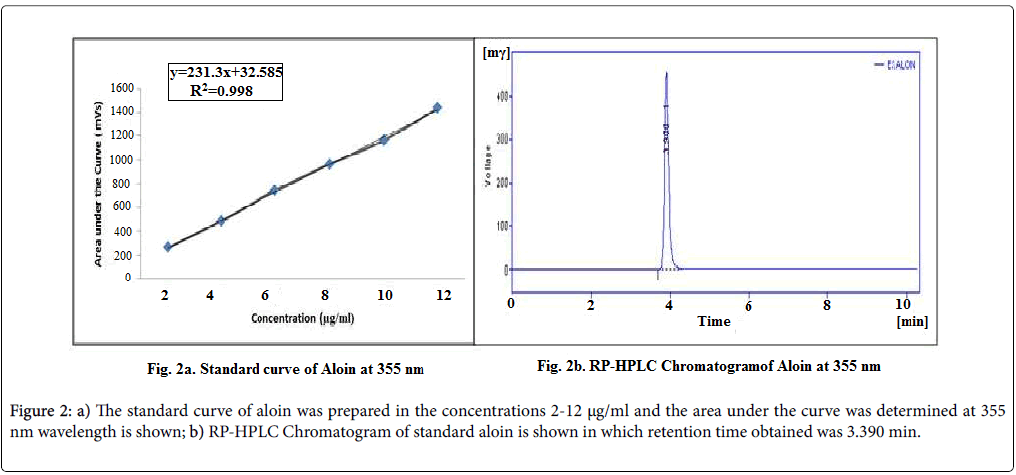
The entrapped aloin was determined by the High performance liquid chromatography using used acetonitrile-water (30:70) as the mobile phase with a flow rate 1.0 mL/min. The standard curve of aloin was prepared in the concentrations 2-12 μg/ml and the area under the curve was determined at 355 nm wavelength as shown in Figure 2a. RP-HPLC Chromatogram of standard aloin is shown in Figure 2b in which retention time obtained was 3.390 min. With the help of this curve as standard the content of aloin in the A. vera extract was assessed as 16.542 ± 0.364 % w/w. By taking this curve as the standard the concentration of aloin in the A. vera extract loaded formulations was determined.
Development of herbal extract loaded ethosomes
Ethosomes were prepared by the cold method. Ethanol is an established efficient permeation enhancer and is present in a quite high concentration (20-50%) in ethosomes. However, due to the interdigitation effect of ethanol on lipid bilayers, it was commonly believed that vesicles could not coexist with high concentration of ethanol.
The high concentration of ethanol (20-50%) in ethosomal formulation could disturb the skin lipid bilayer organization. Therefore, when integrated into a vesicle membrane, it could give an ability to the vesicles to penetrate the stratum corneum. Furthermore, due to high ethanol concentration the ethosomal lipid membrane is packed less tightly than conventional vesicles but possess equivalent stability. This allows a softer and malleable structure, giving more freedom and stability to its membrane, which could squeeze through small openings created in the disturbed stratum corneum lipids. In addition, the vesicular nature of ethosomal formulations could be modified by varying the ratio of components.
The A. vera extract loaded ethosomes (AE1, AE2 and AE3) were prepared with 1%, 1.5% and 2.0% of A. vera extract by taking 2 gm. of soya phosphatidyl choline, 20% of ethanol, 80% of distilled water and sonicated for 5 min. The prepared ethosomal vesicles were evaluated for entrapment efficiency, vesicular size, polydispersity index and zeta potential as depicted in Table 3.
| Formulation Code | Extract concentration % w/w | Entrapment Efficiency % | z-average mean (nm) ± S.D |
Polydispersity index | Zeta potential (mV) |
|---|---|---|---|---|---|
| AE1 | 1.0 | 68.5 ± 1.0 | 175.8 ± 2.0 | 0.273 ± 0.022 | -30.8 ± 1.2 |
| AE2 | 1.5 | 70.8 ± 1.5 | 184.6 ± 1.5 | 0.252 ± 0.012 | -31.5 ± 1.6 |
| AE3 | 2.0 | 71.6 ± 1.5 | 190.5 ± 2.5 | 0.232 ± 0.056 | -32.6 ± 2.0 |
| SD is standard deviation, measurements are taken thrice (n=3).P<0.05 | |||||
Table 3: In vitro evaluation parameters of A. vera extract loaded ethosomes.
The surface charge of A. vera extract loaded ethosomes was negative, ranging from -30.8 ± 1.2 to -32.6 ± 2.0 mV. However, zetapotential values of ethosomes were more negative than liposomes, owing to the presence of ethanol that can impart a negative charge to the vesicles. The incorporation of extract caused a significant (P<0.05) change in the vesicular size and zeta potential.
Morphological determination of herbal extract loaded ethosomes
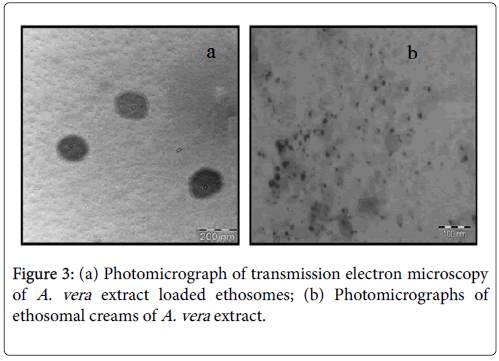
During the development of herbal extract loaded ethosomes various parameters were changed and the vesicles were viewed by microscope for their morphological characters in which the samples with circular shape and dense vesicles were selected. The samples showing aggregation or clumping or tailing of vesicles were rejected. Later on they were viewed by transmission electron microscopy (TEM) by negative staining in which actual size and shape was confirmed. As shown in photomicrographs of transmission electron microscopy (TEM) vesicular structure was confirmed (Figure 3a).
Morphology of the novel vesicles present in cream
By the photomicrographs of transmission electron microscopy (TEM) of creams the presence of vesicles was confirmed in the creams which was to authentify the procedure that after incorporating into the creams the vesicles were not disrupted they maintained their circular structure (Figure 3b). This is just a preliminary evaluation; the actual presence could be indicated by the activity of the prepared novel vesicular creams on skin which will be assessed by bioengineering studies and biological studies discussed.
Physicochemical evaluations of cream formulations
Several physicochemical parameters were measured for each prepared cream formulation according to the Indian Standard Bureau methods. These physicochemical parameters provided information regarding formula stability and skin compatibility. Based on the physicochemical parameters shown in Table 4 lower acid and saponification values, high thermal stability, resulted in stable formulations. The results were similar to that of the results obtained by Ahshawat et al. [38] and Kapoor and Saraf [39]. Key chemical parameters that must be controlled are ash value, acid values, fatty content, non-volatile content and pH. Overall the pH of herbal extract loaded vesicular cream was in the range which is compatible with the pH of the skin. Most of the literature support that the pH of the normal skin is between 5.4 and 5.9. The formulations near this pH don’t cause any adverse effects to the skin and are skin compatible.
| Formulation Code | pH | Non-volatile (% ) | Saponification value | Acid value | Fatty Concentration (% w/w) | Spreadability (% ) | Layer thickness (µm) | Ash value | Viscosity (Cps) |
|---|---|---|---|---|---|---|---|---|---|
| BC | 5.83 ± 0.01 | 14.91 ± 0.5 | 24.0 ± 0.5 | 6.73 ± 0.3 | 15.8 ± 0.9 | 90 ± 2 | 6.73 ± 0.3 | 0.06 ± 1 | 5967 ± 60 |
| AC1 | 5.65 ± 0.03 | 20.43 ± 0.1 | 25.12 ± 0.4 | 5.43 ± 0.2 | 13.23 ± 0.4 | 93 ± 1 | 5.25 ± 0.2 | 0.02 ± 2 | 5884 ± 20 |
| AC2 | 5.46 ± 0.04 | 19.12 ± 0.3 | 26.68 ± 0.3 | 4.32 ± 0.5 | 12.26 ± 0.2 | 94 ± 3 | 5.03 ± 0.1 | 0.03 ± 3 | 5720 ± 10 |
| AC3 | 5.35 ± 0.03 | 18.7 ± 0.2 | 27.35 ± 0.2 | 3.76 ± 0.3 | 12.20 ± 0.3 | 95 ± 2 | 5.18 ± 0.2 | 0.01 ± 3 | 5680 ± 20 |
| EC0 | 6.01± 0.02 | 19.9 ± 0.5 | 38.12 ± 0.4 | 10.15 ± 0.2 | 14.8 ± 0.6 | 96 ± 2 | 10.15 ± 0.2 | 0.01 ± 1 | 5926 ± 20 |
| AEC1 | 5.85 ± 0.03 | 18.6 ± 0.2 | 39.14 ± 0.3 | 6.46 ± 0.1 | 14.34 ± 0.2 | 96 ± 1 | 7.04 ± 0.3 | 0.02 ± 2 | 5810 ± 10 |
| AEC2 | 5.76 ± 0.01 | 17.2 ± 0.3 | 39.48 ± 0.1 | 7.32 ± 02 | 13.26 ± 0.4 | 97 ± 2 | 7.56 ± 0.2 | 0.04 ± 1 | 5770 ± 15 |
| AEC3 | 5.52 ± 0.04 | 16.53 ± 0.2 | 39.63 ± 0.5 | 7.25 ± 0.1 | 12.45 ± 0.1 | 98 ± 3 | 8.34 ± 0.3 | 0.03± 2 | 5680 ± 10 |
| SD is standard deviation, measurements are taken thrice (n=3),P<0.001 as compared to base area | |||||||||
Table 4: Physicochemical evaluations of cream formulations of A. vera extract.
Cell culture reports
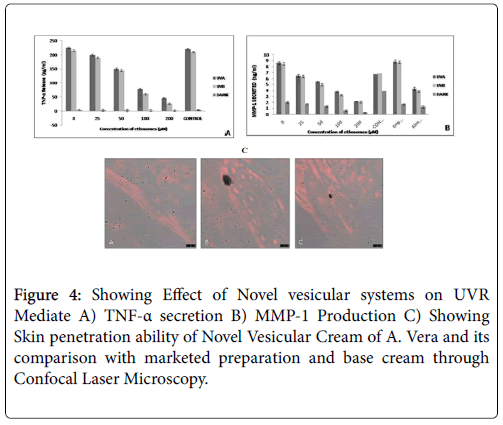
The ability of novel vesicular system to prevent UVA and UVB induced production and/or release of MMP-1 and TNF-α was previously evaluated in cultured keratinocyte mono-layers. Our objective was to evaluate the targeting potential of novel vesicular system of A. vera against UV exposure in human epidermal cell lines. The novel vesicle of A. vera was found significant in inhibiting UV radiation induced cellular deformation. In our experiment, the HaCaT cell cultures were dosed with appropriate amounts of novel vesicular systems and incubated for 24 h prior to exposure to UVA or UVB, and then the media was removed. This was done to observe and evaluate the photo protective as well as targeting ability of the vesicles during dosing period. The results reveals that the inhibitory potential of A. vera ethosomes could be employed further for the purpose of targeting and the action exerted by the vesicular system may be due to their inherent property of an anti-oxidation, photo-absorption tendency, or an enzyme modulating property (Figure 4A).
As can be seen from Figure 4B, pre-treatment of HaCaT cells with 100 μM and 200 μM novel vesicular systems in media 24 h prior to UVR exposure resulted in a significant decrease in the amount of MMP-1 released by the cells relative to both vehicle control (DMSO) and untreated cells. This significant decrease in MMP-1 release is seen in both UVA and UVB exposed cells, though the decrease is more dramatic in the UVA exposed cells. Pre-treatment of the HaCaT cells with 50 μM novel vesicular system 24 h prior to UVR exposure shows a trend towards a decrease in MMP-1 release in both UVA and UVB exposed cells, but this decrease did not reach the level of significance due to high variability in the vehicle control and untreated samples.
Skin penetration study of novel vesicular cream of A. vera
The confocal laser microscopy study was employed to study the skin penetration effect Novel Vesicular Cream of A. vera using goat skin invitro . Goat skin was employed for the purpose of evaluating skin penetrating ability of formulated conjugates because the goat’s skin is anatomically and physiologically similar to that of human skin. The skin penetration of efficacy of novel vesicular cream of A. vera was studied in comparison to the base cream using Rhodamine 123. Briefly, the test samples and the probe containing 0.03% of rhodamine were applied homogeneously and non-occlusively to the skin. The experiments were carried out employing Franz diffusion cells with the receiver chamber filled with phosphate buffer pH 5.5 solutions. After 24 h, the skin was removed and washed with phosphate buffer. The skin was then rapidly frozen by liquid nitrogen and a skin surface perpendicular rectangular piece was taken from the site of drug application with the help of a sharp blade. This tissue was fixed on the sample holder with the help of a Tissue frozen medium gel. (Gung, Leica, Germany). The skin perpendicular sections (dermis to horny layer) of (250 μm) full thickness were cut with the help of cryomicrotome (Leica, Germany). The treated area was cut out and tested for probe penetration. The full skin thickness was optically scanned at 15-30 nm increments through the Z-axis of a Leica DMIRE2 confocal laser scanning microscope (Germany) attached to a Leica TCS SP2 fluorescence microscope (Figure 4C). The figures are shown below.
Biochemical analysis
The antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase constitute a mutually supportive team of defence against Reactive Oxygen Species (ROS). Endogenous antioxidant enzymes are responsible for preventing or neutralizing the free radical induced damages of tissues. The increase in MDA level in skin tissues suggests enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defence mechanisms to prevent formation of excessive free radicals. Pre-treatment with cream formulations significantly reversed these changes [32].
The skin provides natural defence against the oxidative stress caused by deleterious ultraviolet radiations as the epidermis constitutes reactive oxygen species (ROS) detoxifying enzymes (Superoxide dismutase, catalase, thioredoxinreductase and glutathione reductase and low molecular mass antioxidant molecules like glutathione, alpha tocopherol and ascorbic acid. With the initiation of photo oxidative reactions several mechanisms occur like damage of biomolecules, activation of protein kinase C, depletion of antioxidant defence and induction of Ornithine decarboxylase and COX-2 activities. The Protein kinase C activation leads to increase in reactive oxygen species which react with proteins, lipids and DNA and causes formation of cyclobutane pyrimidine dimers. Skin tries to prevent the damages by its self-defence up to a certain level but after that additional antioxidant active constituents are required to fight against these deleterious effects. Catalase is an enzyme that scavenges H2O2 in the skin and provides a buffer action against free radicals generated as a result of UV exposure, in an earlier study by Kaur and Saraf [5].
The measurements of biochemical parameters were done initially without the application of any formulation or Ultra violet (UV) radiation, this group was called as Control. For the control the value of Superoxide dismutase was 59.69 ± 3.16, Malondialdehyde was 1.2 ± 0.14 mM/mL, Ascorbic Acid was 4.09 ± 0.11 mg/100 mL, Catalase was 32.68 ± 2.75 (mM of H202 utilized l min/mg) and total protein was 561.37 ± 3.09 μg mL) as depicted in Table 16. After the UV radiation application the Superoxide dismutase was reduced to 40.05 ± 3.50, Malondialdehyde was increased to 1.96 ± 0.27 mM/mL, Ascorbic Acid was reduced to 1.77 ± .017 mg/100 mL, Catalase decreased to 21.99 ± 2.84 (mM of H202 utilized l min/mg) and total protein was 479.57 ± 9.72 μg mL. The biochemical analysis results show that various biochemical constituents of skin are significantly affected by the effect of ultraviolet radiations. There were 30 ± 5% reductions in the biochemical constituents. In the table of biochemical estimations (Table 5) the values of Control, UV treated and UV treated after empty vesicular cream formulations are same. The changes are from extract loaded formulation. But we have included them in each table for the ease of comparison.
| Group details | Superoxide dismutase (Percentage inhibition of NBT reduction) | MalondialdehydemM/mL | Ascorbic Acid mg/100 mL | Catalase (mM moles of H202 utilized l min./mg) | Total protein (ug mL) |
|---|---|---|---|---|---|
| Control | 59.69 ± 3.16 | 1.2 ± 0.14 | 4.09 ± 0.11 | 32.68 ± 2.75 | 561.37 ± 3.09 |
| UV treated | 40.05 ± 3.50 | 1.96 ± 0.27 | 1.77 ± .017 | 21.99 ± 2.84 | 479.57 ± 9.72 |
| UV+BC | 42.45 ± 2.24 | 1.8 ± 0.07 | 1.88 ± 0.24 | 22.32 ± 1.23 | 484.57 ± 8.34 |
| UV+EC0 | 45.86 ± 2.32 | 1.70 ± 0.02 | 2.12 ± 0.10 | 24.43 ± 1.69 | 499.14 ± 6.96 |
| UV+NC0 | 44.74 ± 2.06 | 1.72 ± 0.01 | 2.08 ± 0.08 | 23.98 ± 1.20 | 496.28 ± 2.34 |
| UV+AC3 | 45.06 ± 1.16 | 1.60 ± 0.02 | 2.05 ± 0.14 | 24.54 ± 1.26 | 497.25 ± 2.09 |
| UV+AEC3 | 52.15 ± 2.18 | 1.43 ± 0.16 | 2.85 ± 0.15 | 31.24 ± 2.18 | 526.56 ± 2.02 |
| n=3, M ± SD (M=mean, SD=standard deviation), P<0.001 as compared to baseline CC) UV:Ultra Violet radiations; E:Ethosomes; C:Cream; BC:Base Cream; Control is without formulation application or without UV radiation application (Base line value) |
|||||
Table 5: Biochemical estimations of A. vera extract loaded formulations.
By the application of base cream (BC) the level of superoxide dismutase was enhanced up to 42.45 ± 2.24, Malondialdehyde level became 1.8 ± 0.07 mM/mL, ascorbic acid level increased to 1.88 ± 0.24 mg/100 mL, catalase level was 22.32 ± 1.23 and total protein content increased to 484.57 ± 8.34 μg/mL. 6 to 8% of improvement in all the biochemical parameters was observed with the base cream. As base cream has its own active constituents which themselves possess distinct properties, hence the use of base cream will enhance the photo protective ability of the prepared herbal extract loaded vesicles.
By the application of empty ethosomal creams (EC0) the level of superoxide dismutase was enhanced to 45.86 ± 2.32, Malondialdehyde level decreased to 1.70 ± 0.02 mM/mL, ascorbic acid level increased to 2.12 ± 0.10 mg/100 mL, catalase level was 24.43 ± 1.69 and total protein content increased to 499.14 ± 6.96 μg/mL.
Further, on the application of the herbal extract loaded ethosomal cream highly significant (P<0.001) improvement was observed. The plain extract loaded conventional creams also produced significant changes. Among the conventional creams the highest improvement was with A. vera extract loaded conventional cream.
As observed from Table 5 as compared to control, superoxide dismutase, ascorbic acid, catalase and total protein content decreased significantly (P<0.001) while Malondialdehyde increased when the ultraviolet radiations were exposed to the skin. Further on application of the prepared formulations before ultraviolet radiations the effect of the radiations was reduced. The improvement was observed highly significant (p<0.001) with extract loaded ethosomal cream formulations than with plain extract loaded creams (p<0.05) as compared to direct ultraviolet radiations treated skin. The increase in extract concentration also enhanced these effects. By the pre-treatment with the prepared formulations the reversal of the biochemical changes caused by the ultraviolet radiations towards normal was observed which confirm the radical scavenging ability of the prepared formulations.
Histological changes
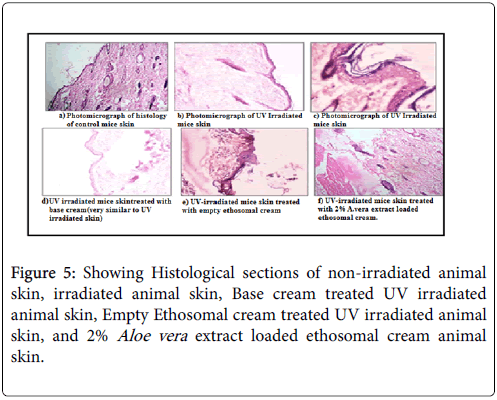
For histological study the animals were divided in five groups i.e. first constitute the histological section of the control group which was not radiated by the ultraviolet radiations. Second consist of the histological section of group second which was exposed to UV radiations, Third is the group where base cream was employed and Fourth were those section were empty ethosomal cream was applied. Fifth constitute the histological sections of those group which were exposed to UV radiations after application of A. veraethosomal cream. The histological slides were prepared by staining the skin tissues with haematoxylin and eosin and the changes in the skin epidermis and dermis along with various cellular structures of the skin were studied. The Figure 5a shows all the skin cellular structures like hair follicles, elastin fibers, collagen fibers, connective tissues all were clearly visible with intact outer layer of epidermis and the inner dermis. In the ultraviolet radiation treated skin (Figures 5b and 5c) epidermal hyperplasia, infiltration of the skin layers, destruction of the integrity of the connective tissue with discontinuous and fibroblastic collagen bundles could be seen in comparison to control (untreated) skin. This might be due to the generation of excess free radicals by ultraviolet radiations which react with cellular lipids, proteins and nucleic acids, leading to degeneration of the skin. The histopathological hall-mark of photo-aging is a massive accumulation of elastotic material in the upper and mid dermis also known as solar elastosis. Elastosis is due to the accumulation of damaged elastin, the main component of elastic fibers, and is associated with degeneration of the surrounding collagen meshwork.
In (Figures 5d and 5e) the histological section of the group’s third which was exposed to UV radiations after application of base cream. Not very characteristic changes were observed after the application of base cream as well as empty vesicles loaded creams. In Figure 5e, the histological sections which were exposed to UV radiations after application of empty ethosomal cream. The figures were similar to the previous figures which were UV radiated skin. Treatment with base cream and empty vesicles loaded creams showed insignificant changes and was found ineffective to protect the skin damage by chronic UV exposure and the skin section was almost same as in the ultraviolet radiation treated skin. In case of treatment with plain extract loaded creams slight changes in the epidermal skin structure was observed as compared to ultraviolet radiations treated skin, but that was also not very prominent. Figure 5f shows histology after application of A. vera extract loaded cream formulations. In these sections destructions of integrity of connective tissues, infiltration of skin layers and damaged collagen bundles and elastin fibres were observed which were remarkably less in comparison to the above sections.
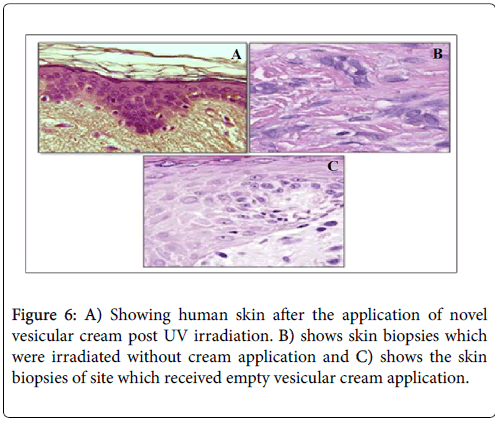
Human biopsies study
The objective behind carrying out this study was to evaluate the inhibitory potentials of A. vera cream against UV radiations in human models. The skin biopsy sections showed that there was a dose dependent effect on human skin in response to the UV radiations (Figures 6A-6C).
Discussion
The concept of internalizing the natural bioactive of A. vera in the form of ethosomal vesicular system is quite a novel perception based on the traditional approach to treat skin related problems. There have been various studies done on ethosomes in which they have been utilized as a carrier for conventional as well as herbal drugs. Ethosomes have been remained a choice in different dermatological formulation, for example, encapsulated fluconazole encapsulated ethosome in an appropriate base to treat fungal diseases topically. They found that ethosomal formulation was far more significant in activity as compared to the liposomal formulation of the same drug. With the same objective to enhance the drug action and its penetration ability, formulation of Tacrolimus-Loaded Ethosomes is done for dermal delivery and found that ethosomal preparation have higher entrapment efficiency with high stability too. In case of herbal drugs, Chen Jin-Guang et.al successfully entrapped curcumin in ethosomal vesicles and found that it increased its percutaneous permeability, entrapment efficiency and therapeutic efficacy [40]. They further observed that the concentration of ethanol and phospholipids greatly interferes with particle size and its entrapment efficiency. Similarly in resemblance to the above studies, we found that ethosome can remarkably deliver the phyto-constituents deeper to the inner core of skin. The Aloe vera ethosomes prepared by us showed a good entrapment efficiency of 71.6 ± 1.5%, a zeta potential of -32.6 ± 2.0 and an average size of 190.5 ± 2.5 nm. Further, the TEM study also confirmed its uniform morphological characters. (Figures 3a and 3b). In the course of experimentation, various studies were carried out like physiochemical evaluation which scale ups about the general parameters of the cream like pH, Non-volatile content, saponification value, acid value, fatty acid concentration, spread ability, layer thickness etc.
In order to estimate the cellular damage caused by UV radiations, two biomarkers of UVR induced damage were employed. Firstly, MMP-1, which is a marker of ROS mediated damage and photo aging effects and TNF-α, a marker of photo-immunosuppression and the causative agent of DNA photo damage. We used these novel vesicular systems in order to determine whether A. vera ethosomes can retard or slow the release of these biomarkers or not. The study showed that in HaCaT cell culture systems novel vesicular systems can reduce UVR induced photo damaging effects. The cell culture systems determined that pre-treatment with Novel vesicular systems 24-132 h prior to UVA or UVB exposure resulted in decreased secretion of MMP-1, as well as on the secretion of TNF-α, which is not reported earlier in topical studies of any other flavanoidal rich novel vesicular system containing formulations. In the case of the HaCaT cell cultures; these ethosomal preparation was dosed into the cell media 24 h prior to UVA and UVB exposure. This dosing protocol allows for absorption of ethosomal vesicular system into the cell prior to exposure to UVA or UVB radiation.
Biochemical analysis like estimation of antioxidant enzymes (super oxidase dismutase, catalase and glutathione peroxidise etc.) were also carried out in accordance with the histological changes occurring in the animal skin. The results of these studies lead to drive the conclusion that the ethosomal formulation is the desired vesicular system for delivering phyto constituents especially when a topical administration is required. This classical vesicular system has the potential to carry a particular phyto-constituent to the desired area of skin, irrespective of the final formulation in which it is formulated.
Human biopsies were taken in order to confirm the activity profile of the novel formulation. The results of skin biopsies study showed that there was a dose dependent reduction in erythema formation. The human skin sections showed a decrease level of inflammation as well as an absence of lesion formation on repeated dosing of UV radiations to thighs skin. No sign of skin sensitivity or allergic reactions were observed on the application of novel vesicular cream. The results also draw our attention towards the fact that, on applying novel vesicular cream on human skin, incidents like epidermal thickening or formation of wrinkles were not observed. The study thus, confirms the role of A. vera ethosomes as a desired vesicles for encapsulating any phyto protectants for dermal application and the vesicular cream as novel photo-protectant. From our study, we came to a conclusion that the ethosomal cream of A. vera , exhibited significant action in reducing skin complications and will be fruitful for its futuristic applications in skin care to develop a good formulation for sunscreen activity.
Acknowledgement
The authors acknowledge the University Grant Commission-SAP [F. No.3-54/2011(SAP-II)] New Delhi, India, and University Grant Commission (UGC) New Delhi, Under MRP Scheme Major Research project, F. No 39-170/2010 (SR), for financial assistance. One of the author extend their gratitude towards the head of the cosmetic lab, University Institute of Pharmacy, Pt., Ravi shankar Shukla University, Raipur (C.G.) for providing facilities to carry out research work.
Conflict of Interest
No conflict of interest associated with this work.
References
- Afaq F, Adhami VM, Ahmad N, Mukhtar H (2002) Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Biosci 7: 784-792.
- Afaq F, Mukhtar H (2006) Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp Dermatol 15: 678-684.
- Ajazuddin, Saraf S (2010) Applications of novel drug delivery system for herbal formulations. Fitoterapia 81: 680-689.
- Ashawat MS, Saraf S (2008) Comparative sun protection factor determination of fresh Aloe vera gel vs marketed formulations. Indian J Pharm Educ Res 42: 319-322.
- Kaur CD, Saraf S (2011) Photoprotective herbal extract loaded nanovesicular creams inhibiting ultraviolet radiations induced photoaging. Int J Drug Deliv 3: 699-711.
- Belo SED, Gaspar LR, Campos PMBGM (2006) Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res Technol 12: 241-246.
- Joseph B, Raj SJ (2010) Pharmacognostic and phytochemical properties of Aloe vera Linn –an overview. Int J Pharm Sci Rev Res4: 106-110.
- Dayan N, Touitou E (2000) Carriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. Liposomes. Biomaterials 21: 1879-1885.
- Kaur CD, Saraf S (2009) Herbal photoprotective formulations and their evaluation. Open Nat Prod J 2: 71-76.
- Deep C, Saraf S (2008) Novel approaches in herbal cosmetics. J Cosmet Dermatol 7: 89-95.
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M (2000) Ethosomes-novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 65: 403-418.
- Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, et al. (2001) Intracellular delivery mediated by an ethosomal carrier. Biomaterials 22: 3053-3059.
- Touitou E, Godin B, Weiss C (2000) Enhanced delivery of drugs into and across the skin by ethosomal carriers. Drug Dev Res 50: 406-415.
- Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM (2006) Deformable liposome and ethosomes: mechanism of enhanced skin delivery. Int J Pharm 322: 60-66.
- Sheer A, Chauhan M (2011) Ethosomes as vesicular carrier for enhanced transdermal delivery of Ketoconazole - Formulation and Evaluation. IJPIs J Pharm Cosmetol 1: 1-14.
- Forster T, Tesmann H (1991) Phase inversion emulsification. Cosmet Toil 11: 106.
- Ahshawat MS, Saraf S, Saraf S (2008) Preparation and Characterisation of herbal creams for improvement of skin viscoelastic properties. Int J Cosmet Sci 30: 183-193.
- Ashawat MS, Saraf S, Saraf S (2007) Antioxidant activity of skin care herbal cosmetic cream and lotion. Plant Arch 7: 685-687.
- Kaur CD, Saraf S (2011) Topical vesicular formulations of Curcuma longa extract on recuperating the ultraviolet radiations damaged skin. J Cosmet Dermatol 10: 260–265.
- Nguyen PLH, Bouwstra JA (2003) The in vitro transport of pergolide from surfactant-based elastic vesicles through human skin: a suggested mechanism of action. J Control Release 86: 145-156.
- Kaur CD, Nahar M, Jain NK (2008) Lymphatic targeting of zidovudine using surface engineered liposomes. J Drug Target 16: 798-805.
- Duangjit S, Opanasopit P, Rojanarata T, Ngawhirunpat T (2011) Characterization and In Vitro Skin Permeation of Meloxicam-Loaded Liposomes versus Transfersomes. J Drug Deliv Pp: 1-9
- Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, et al. (2007) Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release 123: 148-154.
- Cao H, Liu Y (2003) Determination of aloin in aloes by HPLC. Zhongguo Zhong Yao Za Zhi 28: 349-351
- ISO (1997) Methods of test for safety evaluation of cosmetic. BIS (Indian Standard Bureau) IS 4011.
- Colipa (1997) (The European Cosmetic Toiletry and Perfumery Association) Scientific Committee on Cosmetology. Brussels: Notes of guidance for the testing of cosmetic ingredients for their safety evaluation. Pp: 47-48.
- COLIPA (2004) Evaluation of the efficacy of cosmetic products. COLIPA Guidelines. Pp: 1-16.
- Lachapelle JM (1996) Efficacy of protective creams and/or gels: Prevention of contact dermatitis. Curr Probl Dermatol 25: 182-192.
- SCCNFP (Scientific Committee of Cosmetics and Non-Food Products.) (2003) Notes of guidance for testing of cosmetic ingredients and their safety evaluation by the SCCNFP. Pp: 1-102.
- Guyer SF, Afaq F, Mukhtar H (2003) Photochemoprevention of skin cancer by botanical agents. Photodermatol Photoimmunol Photomed 19: 56-72.
- Kapoor S, Saraf S (2009) Age Dependent Studies as Various Skin Parameters Using Cutometer. Indian J Pharm Educ Res 43: 338-345.
- Kaur CD, Saraf S (2011) Skin Care Assessment On The Basis Of Skin Hydration, Melanin, Erythema and Sebum at Various Body Sites. Int J Pharm Pharma Sci 3: 209-213.
- Agrawal R, Kaur IP (2010) Inhibitory effect of encapsulated curcumin on ultraviolet-induced photoaging in mice, Rejuvenation Res 13: 397-410.
- Seite S, Bredoux C, Compan D, Zucchi H, Lombard D, et al. (2005) Histological evaluation of a topically applied retinol-vitamin C combination. Skin Pharmacol Physiol 18: 81-87.
- El-Shemy HA, Aboul-Soud MA, Nassr-Allah AA, Aboul-Enein KM, Kabash A, et al. (2010) Antitumor properties and modulation of antioxidant enzymes activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr Med Chem 17: 129-138.
- Embil K, Nacht S (1996) The Microsponge Delivery System (MDS): a topical delivery system with reduced irritancy incorporating multiple triggering mechanisms for the release of actives. J Microencapsul 13: 575-588.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Ashawat MS, Saraf S, Saraf S (2007) Biochemical and histopathological studies of herbal cream against UV radiation induced damage. Trends Med Res 2: 135-141.
- Kapoor S, Saraf S (2009) Efficacy study of sunscreens containing various herbs for protecting skin from UVA and UVB sunrays. Phcog Mag 5: 238-48.
- Jin-guang C, Wei L, Yu J (2013) Preparation of curcumin ethosomes. Afr J Pharm Pharmacol 7: 2246-2251.
Citation: Kaur CD, Gupta A, Saraf S (2018) Effect of Flavanoidal Rich Novel Vesicular Cream on the Cellular Components of Skin Against UV Irradiation. J Cancer Diagn 3: 113. DOI: 10.4172/2476-2253.1000113
Copyright: © 2018 Kaur CD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6699
- [From(publication date): 0-2018 - Nov 12, 2025]
- Breakdown by view type
- HTML page views: 5735
- PDF downloads: 964