Research Article Open Access
Effect of Weight Loss on Markers of Inflammation and Endothelial Function in Childhood Obesity
Iezzi ML1*, Bruzzi P2, Lasorella S1, Predieri B2, di Pianella AV1 and Iughetti L21Paediatric Department, San Salvatore Hospital, University of L’Aquila, L’Aquila, Italy
2Department of Medical and Surgical Sciences of Mothers, Children and Adults, Pediatric Unit, University of Modena & Reggio Emilia, Modena, Italy
- *Corresponding Author:
- Maria Laura Iezzi
Paediatric Department, San Salvatore Hospital
University of L’Aquila, L’Aquila, Italy
Tel: + 3388544569
E-mail: marialaura.iezzi@libero.it
Received date: January 04, 2017; Accepted date: February 06, 2017; Published date: February 08, 2017
Citation: Iezzi ML, Bruzzi P, Lasorella S, Predieri B, di Pianella AV, et al. (2017) Effect of Weight Loss on Markers of Inflammation and Endothelial Function in Childhood Obesity. J Obes Weight Loss Ther 7:333. doi: 10.4172/2165-7904.1000333
Copyright: © 2017 Iezzi ML, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: Obesity is associated with chronic low-grade inflammation and hyperinsulinism that may influence the progression of endothelial dysfunction and atherosclerosis already in childhood. Methods: To study changes in metabolic profile and markers of inflammation and endothelial activation in children with primary severe obesity after weight loss we involved 14 obese children (Ob) that underwent a lifestyle intervention and 18 normal weighted subjects (C). In Ob, anthropometric data were assessed both at baseline and after intervention together with oral glucose tolerance test and fasting evaluation of cholesterol assessment, interleukin-6, endogenous secretory receptor of advanced glycation end products and endothelin levels. Results: At baseline, serum IL-6 concentrations resulted significantly higher in Ob respect to C (12.96 ± 8.87 vs. 4.88 ± 1.19 pg/ml, p< 0.05). After weight loss, Ob significantly improved glucose metabolism and lipid assessment and they showed a significant reduction of all markers of inflammation and endothelial activation. In all subjects studied, BMI-SDS correlated positively with interleukin-6 (r 0.45, p < 0.05). Conclusion: Our results demonstrated higher concentrations of inflammatory markers in obese children compared to healthy subjects. Nevertheless, an early lifestyle intervention could improve the levels of these molecules together with cholesterol and glucose metabolism and may reverse the development of premature endothelial dysfunction in obese children.
Keywords
Children; Inflammation markers; Life style intervention; Obesity; Weight loss
Introduction
Obesity is a major public health problem in industrialized countries [1] A recent survey demonstrates that 21-24% of American children and adolescents are overweight and 16-18% obese [2]. A similar epidemic condition is undergoing in Italy, where overweight and obesity affect nearly 40% of children in Central and Southern regions [3]. Childhood and adolescent obesity is predictive of adult obesity, as previously documented [4]. Overweight and obesity are associated with hyperinsulinism and insulin resistance with an increased incidence of dyslipidemia, type 2-diabetes and cardiovascular disease already in childhood and adolescence; in particular, it has been shown that severe childhood obesity is independently associated with endothelial dysfunction and arterial wall thickening [5]. These pathological conditions can persist in adulthood regardless of the maintaining of excessive weight. Recent studies show that obesity is a status of chronic inflammation characterized by the production of cytokines by adipose cells [6]. It has been shown that adipose tissue cells (adipocytes and macrophages) secrete a cascade of proinflammatory cytokines, including interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), interleukin-1 (IL-1) and interleukin 10 (IL-10), as well as reagents oxygen and endothelial adhesion molecules with a paracrine and systemic effect. This up-regulation of endothelial adhesion molecules plays a basic role during the earliest phases of atherogenesis and it causes dysfunction and activation of endothelial cells, then promoting abnormal adhesion of leukocytes and platelets and forming the basis of atherosclerotic plaque [7,8]. This low-grade of persistent inflammation, associated with hyperinsulinemia may influence the progression of endothelial dysfunction and it could be responsible for the beginning of early obesity-associated atherosclerosis in childhood Thus, obesity per se could promote atherosclerotic plaque formation since the earliest ages and it could be considered a strong predictor of coronary heart disease later in life [9]. The primary objective of the pediatrician must be to develop effective strategies to early identify and treat diseases related to obesity in children [10].
The aim of the present prospective and longitudinal study was to investigate the changes of endothelial adhesion molecules and inflammatory cytokines in severe obese patients before and after loss weight.
Materials and Methods
Study population
The study included Italian children and adolescents with severe obesity and normal weight subjects, matched according to age, gender and pubertal stage, attending the Pediatric Metabolic Disorders Clinics of the University of Aquila and of the University of Modena and Reggio Emilia, Italy, recruited between January and September 2015.
Exclusion criteria consisted of concomitant endocrinological diseases and use of medications affecting insulin sensitivity or glucose/ lipid metabolism.
Obese children underwent to a 1-year long lifestyle intervention: a gradual change in eating habits was proposed to the whole family (appropriate balance of nutrients and correct distribution of meals in 5 times of the day, avoiding snacks, and restoring importance of breakfast) and sedentary lifestyles were reduced increasing physical activity. The food portions were estimated through simple benchmarks in daily habits.
In Ob group, anthropometric data, biochemical parameters, serum concentration of IL-6, endothelin 1(ET-1) and endogenous secretory receptor of advanced glycation end products (esRAGE) were determined both at baseline (time 0) and after intervention (time 1).
Written informed consent for data collection was obtained from all subjects and parents at the moment of recruitment in the study and before the first data collection. The approval of the local Ethics Committees was obtained.
Body composition and definition of obesity
All patients underwent a complete clinical history and anthropometric measurements that were performed by well-trained examiners according to the Anthropometric Standardization Reference Manual. Height was measured to the nearest 0.1 cm using a wallmounted stadiometer (Harpenden, Crymych; UK). Body weight was measured to the nearest 0.1 kg and body mass index (BMI) was calculated as weight in kg/height in meters squared and expressed as zscore with respect to chronological age using national growth charts (Cacciari 2006). Obesity was defined as BMI higher than the 95th percentile for age and sex, severe obesity when BMI was higher 99th percentile, whereas normal weight was defined as a BMI lower than the 85th percentile.
Laboratory measurements
Fasting lipid profile [total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG)] was evaluated using the commercially available kits for enzymatic test (Roche Diagnostics, Mannheim, Germany) which has international consensus values for plasma lipids, ensuring that magnitude of the potential difference is acceptable at 10%. The sensitivity of the TC, HDL-C, and LDL-C assay was 3 mg/ml. Intra and inter assay coefficients of variations (CVs) were less than 0.8% and 1.7%, respectively, for all examinations. The sensitivity of the TG assay was 4 mg/ml, while intra- and inter assay CVs were 1.5% and 1.8%, respectively.
Oral glucose tolerance test (OGTT) was performed at baseline and at the end after the beginning of the lifestyle intervention. The glucose load for OGTT was 1.75 g/Kg (maximum administered dose 75 g). The categorization of glucose tolerance status was made using the World Health Organization criteria.
Blood samples to measure inflammatory markers were obtained after a 12-h fasting period and centrifuged at 3000 g at 4°C for 10 min to obtain serum. Samples were frozen at −80°C until assayed.
ELISA (enzyme-linked immunosorbent assay) method was used to detect esRAGE (esRAGE ELISA Kit; B-Bridge International Inc., CA, USA) serum levels. The esRAGE concentrations were measured according to user manual and they were determined by interpolation of the regression curve formula. The sensitivity of the assay was 0.05 ng/ml. No significant cross-reactivity or interference was observed in these assays.
ET-1 serum concentrations were assayed using enzymatic kits (TiterZyme®, Assay Designs Inc., MI, USA). The sensitivity of the assay was 0.14 pg/ml. The intra assay CVs according to low, medium and high concentrations of ET-1 were 8.5%, 5.8%, and 4.6%, respectively; the inter assay CVs were 8.1%, 4.7%, and 3.1%, respectively.
IL-6 levels were determined by ELISA method using the Quantikine kit (R&D System), the minimum detectable concentration was 0.10 pg/ml and the interassay CV was 7.0%.
Statistical analysis
All normally distributed variables (BMI, z-score BMI) were expressed as mean ± SD. Data not normally distributed (TC, LDL-C, TG, HDL-C, glycaemia and insulin at baseline and at 120 minutes after OGTT, ET-1, IL-6, esRAGE) were log-transformed to approximate normality and expressed as mean ± SD. Between group comparison of anthropometric and biochemical data at time 0 were evaluated using Mann-Whitney’s U-test. In Ob group, longitudinal changes of anthropometric, biochemical, and inflammatory data were analyzed using the Wilcoxon matched pair test. Spearman’s correlation analysis was performed to assess the relationship between variables. The association between potential predictors and longitudinal changes of inflammatory markers was evaluated using the multiple linear regression model including Δ ET-1, Δ IL-6, and Δ esRAGE as dependent variables and anthropometric and biochemical data (with an association with the dependent variable in univariate analysis at 15% significance level) as independent variables. Statistical significance was inferred at a p value of <0.05.
Results
Baseline data
Thirty two subjects entered the study: 14 children and adolescents with severe obesity [group Ob, 6 boys and 8 girls, aged 11.0 ± 0.10 years, body mass index (BMI)>99th percentile) and 18 normal weight subjects (group C, 9 boys and 9 girls, aged 10.4 ± 3.64 years, BMI <85th percentile); baseline data of group Ob and group C patients are outlined in Table 1.
| Patients | Controls | |
| Age (years) | 11.00 ± 0.10 | 10.40 ± 1.64 |
| BMI (kg/m2) | 28.53 ± 3.38 | 17.14 ± 2.34* |
| BMI SDS | 2.90 ± 0.44 | 0.17 ± 0.91* |
| ET1 (pg/ml) | 9.35 ± 4.57 | 6.13 ± 4.25 |
| IL-6 (pg/ml) | 12.96 ± 8.87 | 4.88 ± 1.19* |
| esRAGE(ng/ml) | 0.37 ± 0.11 | 0.51 ± 0.28 |
| Legend: *p<0.05 between groups | ||
Table 1: Baseline features in study population.
At time 0 IL-6 levels were significantly higher in Ob group than in C group (12.96 ± 8.87 vs. 4.88 ± 1.19 pg/ml, respectively; p=0.002; Table 1) while ET-1 and esRAGE concentrations were not significantly different.
Longitudinal data
All patients of Ob group were longitudinally evaluated at the end of the study.
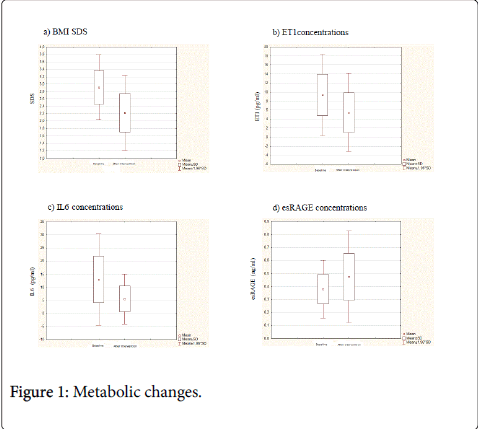
Weight loss (Figure 1a) and the improvement of both glycaemic control and lipid profile were longitudinally demonstrated in our Ob group (Table 2). Specifically, the lifestyle intervention treatment from time 0 to time 1 significantly decreased z-score BMI (2.90 ± 0.44 vs. 2.22 ± 0.52 SDS, respectively; p<0.001), TG levels (90.92 ± 38.50 vs. 65.71 ± 17.72 mg/dl, respectively; p<0.001), both glycaemia (103.61±7.80 vs. 86.91±15.20 mg/dl, respectively; p=0.005) and insulin (84.77 ± 39.56 vs. 55.21 ± 24.50 microIU/ml, respectively; p=0.01,) at 120 minutes after OGTT. HDL-C values increased significantly from 40.78 ± 5.88 to 50.14 ± 15.67 mg/dl (p=0.04). The other parameters of lipid profile did not change despite intervention (Table 2).
| Time 0 | Time 1 | |
| BMI (kg/m2) | 28.53 ± 3.38 | 25.65 ± 3.68° |
| BMI SDS | 2.90 ± 0.44 | 2.22 ± 0.52° |
| CT (mg/dl) | 160.92 ± 21.83 | 160.21 ± 19.02 |
| LDL-C (mg/dl) | 101.57 ± 23.45 | 99.71 ± 20.99 |
| HDL-C (mg/dl) | 40.78 ± 5.88 | 50.14 ± 15.67° |
| TG (mg/dl) | 90.92 ± 38.50 | 65.71 ± 17.72° |
| OGTT test: | ||
| Baseline glycaemia (mg/dl) | 77.28 ± 8.52 | 75.85 ± 7.32 |
| 120 min glycaemia (mg/dl) | 103. 61 ± 14.44 | 86.91 ± 15.20° |
| Baseline insulin (uIU/ml) | 17.92 ± 7.80 | 15.64 ± 7.81 |
| 120 min insulin (uIU/ml) | 84.77 ± 39.56 | 55.21 ± 24.50° |
| Legend: °p < 0.05 vs. Time 0 | ||
Table 2: Longitudinal analysis of anthropometric and biochemical data in obese patients.
Together with the previously described metabolic changes a significant decrease of inflammatory markers levels was found (Table 3, Figures 1b-1d). In particular, from time 0 to time 1, ET-1 (9.35 ± 4.57 vs. 5.46 ± 4.41 pg/ml; p<0.001) and IL-6 (12.96 ± 8.87 vs. 5.60 ± 4.91 pg/ml; p<0.001) concentrations were significantly decreased; moreover, a significant increase of esRAGE levels (0.37 ± 0.11 vs. 0.47 ± 0.17 ng/ml, p<0.0001) was demonstrated (Table 3). Figure 1 depicts the Δ of inflammatory markers (b, c, and d) along follow-up.
| Time 0 | Time 1 | |
| ET1 (pg/ml) | 9.35 ± 4.57 | 5.46 ± 4.41° |
| IL-6 (pg/ml) | 12.96 ± 8.87 | 5.60 ± 4.91° |
| esRAGE(ng/ml) | 0.37 ± 0.11 | 0.47 ± 0.17° |
| Legend: °p < 0.05 vs. Time 0 | ||
Table 3: Longitudinal analysis of inflammatory markers in obese patients.
At baseline, in the total population, we found a significant and positive correlation between IL-6 concentrations and z-score BMI (r=0.458, p<0.05).
No anthropometric and biochemical variable was identified as significant predictive factor for reduction of ET-1, IL-6 and increase of esRAGE in group Ob.
Discussion
In our study lifestyle modifications, including combined diet and moderate regular physical exercise, significantly reduced BMI and zscore BMI and improved insulin sensitivity and lipids profiles. Moreover, together with these metabolic changes, in our Ob group the serum levels of IL-6, ET-1, and esRAGE significantly improved along follow-up.
It has been already shown that IL-6 concentration, normally expressed in white adipose tissue and in peripheral blood mononuclear cells, increases progressively with adiposity [11] and, along with other pro-inflammatory cytokines, may substantially influence obesity development and metabolic disorders such as type 2 diabetes. IL-6 plays a significant role in energetic homeostasis and lipid-carbohydrate metabolism and it is an upstream inflammatory cytokine that plays a central role in propagating the downstream inflammatory pathway responsible for atherosclerosis. In our study, the decrease of IL-6 levels after weight loss indicates that its secretion by the adipocyte can be modulated by lifestyle changes.
The most potent vasoconstrictor peptide synthesized by the endothelial cells is ET-1 and it appears to play a central role in the pathophysiology of the vasomotor abnormalities associated with endothelial dysfunction; moreover, it is involved in the progress of the atherosclerotic plaque [12]. It mediates host responses, including endothelial dysfunction, vasomotor contraction, leukocyte and platelet activation, and cellular proliferation. An increased activity of the ET-1 system probably may contribute to the abnormal vascular homeostasis in obese patients. Endothelial injury is the main stimulus for ET-1 secretion. Children and adolescents with obesity, hypertension or diabetes have higher ET-1 plasma concentrations than healthy subjects, and its level correlates with BMI, lipid parameters and systolic blood pressure [13]. Our data confirm published data but it is important to underlie how in our Ob subjects ET-1 levels significantly decreased after weight loss due to the only lifestyle intervention.
During these last years, both the soluble secretory receptors for advanced glycation end products (sRAGE) and the esRAGE were demonstrated to be involved in the pathogenesis of cardiovascular diseases [14]. sRAGE is a multi-ligand cell-surface protein, belonging to the immunoglobulin superfamily. RAGE interacts with other nonglycated peptide ligands, including S-100/calgranulin, amphoterin, amyloid fibrils and a leukocyte integrin, macrophage-1 antigen (Mac-1). Ligand engagement of RAGE leads to prolonged inflammation, and is also profoundly associated with macrovascular complications in diabetes due to the expression of pro-inflammatory mediators such as monocyte chemoattractant protein 1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1). Recently, numerous truncated forms of RAGE have been described; they are spliced variants of RAGE, which carry all of the extracellular domains but are lacking of the trans-membrane and intracytoplasmic domains. They bind ligands including advanced glycation end products (AGEs) and can antagonize RAGE signaling in vitro and in vivo. A C-terminally truncated soluble form of RAGE has received much attention; it is now known to be present in human circulation and is named esRAGE. esRAGE neutralizes the effects of AGEs on endothelial cells in culture. Thus, the decoy function of esRAGE may involve a feedback mechanism by which esRAGE prevents the activation of RAGE signaling. It has also been suggested that some esRAGE isoforms that could act as decoy receptors, may be cleaved proteolytically from the native RAGE expressed on the cell surface [14,15].
In our population, we found a significant increase of esRAGE levels after the weight loss due to lifestyle treatment. The effectiveness of therapeutic lifestyle changes in improving or preventing the components of the metabolic syndrome has already been demonstrated in adults [16], but only few studies demonstrated that weight reduction resulted in an improvement of the metabolic syndrome and of the atherogenic risk-factor profile in children. A positive association between HOMA-IR, waist circumference (WC), lipid profile, and BMI was found [17], while the few studies evaluating the impact of weight loss on serum markers of systemic inflammation in obese children reported controversial results. In one study, the prevalence of metabolic syndrome decreased in adolescents if they followed a better overall Healthy Eating Index and if they trained more physical activities [18].
Furthermore, in particular, only few previous studies evaluated the impact of weight loss of markers of systemic inflammation levels in obese children (Table 4).
| Authors | Molecules in study | Intervention | Age of patients (year) | Number of patients | Results |
| Gong l et al. | IL-6 | 1 year of lifestyle modification | 7-11 | 326 | Reduction of IL-6 conc. |
| D’adamo et al. | esRAGE | 6 month lifestyle intervention with Vitamin E supplementation | 8.3 ± 1.6 8.4 ± 1.3 |
42 | Increased of esRAGE |
| Nemet et al. | IL-6 | 3 months dietary intervention and exercise program | 10.4 ± 1.96 | 21 | No change in IL-6 |
| Roberts et al. | IL-6 | 2 week program oflow-fat diet and daily exercise program | 13.1 ± 0.5 | 19 | Reduction of IL-6 conc. |
| Rynders et al. | IL-6 | 6 months of hypocaloric diet, exercise, and metformin | 14.3 ± 2.4 | 16 | Reduction of IL-6 conc. |
| Izadpanah et al. | IL-6 | 2 week residential lifestyle modification | 13.0 ± 0.5 | 21 | Reduction of IL-6 conc. |
| Roth et al. | IL-6 | 1 year lifestyle modification | 29 | No change in IL-6 | |
| Garanty-Bogacka et al. | IL-6 | 6-monthhypocaloric diet and moderate physical activity | 14.2 ± 2.6 | 50 | Reduction of IL-6 conc. |
| Martos-Moreno et al. | IL-6 | 18 months of physical activity program and reorganization of eating habits | 8.92 ± 1.80 | 51 | Reduction of IL-6conc. |
| Amati et al. | IL-6 | 6 month hypocaloric diet | 8.9 ± 0.5 | 35 | No change in IL-6 |
| Balagopal et al. | IL-6 | 6 month lifestyle modification | adolescents | 15 | Reduction of IL-6 conc. |
| Gallistl et al. | IL-6 | 3 weeks hypocaloric diet and increase in physical activity | 11.9 ± 1.8 | 49 | Reduction of IL-6 conc. |
Table 4: Review of existing studies in paediatric population.
Among these studies, Gong et al. [19], Roberts et al. [20], Rynders et al. [21], Izadpanah et al. [22], Garanty-Bogacka et al. [23], Martos Moreno et al. [24], Balagopal et al. [25] and Gallistl et al. [26] showed similar change in IL-6 levels after decrease of BMI. On the other side, Nemet et al. [27], Roth et al. [28], and Amati et al. [29] found no significant change. D’Adamo et al. [30] recently demonstrated a significant increase of esRAGE in obese children after life style intervention combined with Vitamin E supplementation.
In two studies [20,21], a medical treatment was added to the diet. In five cases [19,21,24-26], the follow-up lasted only few weeks and it comprises a program of intensive physical activity and hypocaloric diet without including a gradual change in lifestyle intervention and eating habits.
To our best knowledge, no data on the role of ET-1 in children after weight loss is yet available, for these reasons our results are a new developments compared to previous literature.
In childhood, these kinds of programs need the inclusion of a multidisciplinary team. Therefore, they are expensive and, thus, they could be usually conducted for a short period. In addition, most of the proven beneficial effects do not persist once the program is completed. Only in three published studies [17,19,24], the patients were reported to be follow for at least one year and the proposed programs was similar to the one we conducted in our study attempting to modify permanently lifestyle.
The main bias of our study is the small population sample reflecting the complexity to collect longitudinal data over a long-term follow-up period, especially among patients with severe obese that usually are not so compliant to new lifestyle suggestions. Since our study focus attention on IL-6, ET-1 and esRAGE, it could be expanded with evaluation of other chronic inflammation parameters.
Conclusion
Our study demonstrated that weight loss by lifestyle modifications can improve both low-grade inflammation status and insulin resistance in obese children. These changes appear long-term and persistent in children presenting a severe degree of obesity and experiencing a 1 years-long lifestyle intervention that includes dietary and behavioral treatment to achieve a gradual change in the eating habits of the whole family and a reduction of sedentary lifestyles.
In conclusion, we suggest that weight-reduction programme is necessary in pre-pubertal and pubertal obese children with abnormal glycemic and lipid profile as a prevention of metabolic syndrome later in life, since we demonstrated that a non-intensive long-term weight management program may significantly improve the degree of obesity together with glucose metabolism, abnormal lipid profile and some cardiovascular risk factor in childhood.
Informed Consent
Written informed consent for data collection was obtained from all subjects and parents at the moment of recruitment in the study and before the first data collection.
Ethical Considerations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation.
References
- Spiotta RT, MD, Luma GB (2008) Evaluating Obesity and Cardiovascular Risk Factors in Children and Adolescentes. Am Fam Physician 78: 1052-1058.
- Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 307: 483-490.
- Valerio G, Licenziati MR (2012) The course of treatment of children and adolescents with severe obesity. Minerva Pediatr 64: 413-431.
- Simmonds M, Lielellyn A, Owen CG, Woolacott N (2016) Predicting adult obesity from childhood obesity: a sistematyc review and metabolic analysis. Obes Rev 17: 95-107.
- Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, et al. (2010) Childhood Obesity, other Cardiovascular Risk factors, and Prematura death. N Engl J Med 362: 485-493.
- Ross R (1999) Atherosclerosis an inflammatory disease. N Engl J Med 340: 115-126.
- Ferri C, Desideri G (2005) Endothelial activation. Sliding door to atherosclerosis.Curr Pharm Des 11: 2163-2175.
- Morrison JA, Friedman LA, Gray-McGuire C (2007) Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later. The Princeton Lipid Research clinics follow-up study. Pediatrics 120: 340-345.
- Giovambattista D, De Simone M, Iughetti L, Rosato T, Iezzi ML, et al. (2005) Early activation of Vascular Endothelial cells and platelets in obese children. J ClinEndocrinolMetab 90: 3145-3152.
- Iughetti L, De Simone M, Iezzi ML (2008) Thirty-year of persistence of obesity after presentation to a paediatric obesity clinic. Ann Hum Biol 35: 439-448.
- Hartman J, Frishman WH (2014) Inflammation and atherosclerosis: a review of the role of Interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev 22: 147-151.
- Levin ER (1995) Endothelins. N Engl J Med 333: 356-363.
- G┼?owi┼?ska B, Urban M, Hryniewicz A, Peczy┼?ska J, Florys B, et al. (2004) Endothelin-plasma concentration in children and adolescents with atherogenic risk factors. Kardiol Pol 61: 329-338.
- D’Adamo E, Giannini C, Chiavaroli V, de Giorgis T, Verrotti A, et al. (2011) What is the significance of soluble and endogenous secretory receptor for advanced glycation end products in liver steatosis in obese pre-pubertal children? Antioxid Redox Signal 14: 1167-1172.
- Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, et al. (2002) Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation 105: 816-822.
- Stone NJ, Saxon D (2005) Approach to treatment of the patient with metabolic syndrome: lifestyle therapy. Am J Cardiol 96: 15E-21E.
- Kubicky RA, Dunne C, Nandi-Munshi D, De Luca F (2012) Long-term effects of a non-invasive weight program on body mass index and metabolic abnormalities of obese children and adolescents. Int J PediatrEndocrinol 16.
- Pan Y, Prat CA (2008) Metabolic syndrome and it s association with diet and physical activities in US adolescents. J Am Diet Assoc 108: 276-286.
- Gong L, Yuan F, Teng J, Li X, Zheng S (2014) Weight loss, inflammatory markers, and improvements of iron status in overweight and obese children. J Pediatr 164: 795-800.
- Roberts C, Izadpanah A, Angadi S, Barnard RJ (2013) Effects of an intensive short-term diet and exercise intervention: comparison between normal-weight and obese children. Am J PhysiolRegulIntegr Comp Physiol 305: 552-557.
- Rynders C, Weltman A, Del Giorno C, Balagopal P, Damaso L, et al. (2012) Lifestyle Intervention Improves Fitness Independent of Metformin in Obese Adolescents. Med Sci Sports Exerc 44: 786-792.
- Izadpanah A, Barnard RJ, Almeda AJ, Baldwin GC, Bridges SA, et al. (2012) A short-term diet and exercise intervention ameliorates inflammation and markers of metabolic health in overweight/obese children. Am J PhysiolEndocrinolMetab 303: E542-E550.
- Garanty-Bogacka B, Syrenicz M, Goral J, Krupa B, Syrenicz J, et al. (2011) Changes in inflammatory biomarkers after successful lifestyle intervention in obese children. Endokrinol Pol V62: N6.
- Martos-Moreno GA, Barrios V, Martínez G, Hawkins F, Argente J (2010) Effect of Weight Loss on High-Molecular Weight Adiponectin in Obese Children. Obesity 18: 2288-2294.
- Balagopal P, George D, Patton N, Yarandi H, Roberts WL, et al. (2005) Lifestyle-only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. J Pediatr 146: 342-348.
- Gallistl S, Sudi KM, Aigner R, Borkenstein M (2001) Changes in serum interleukin-6 concentrations in obese children and adolescents during a weight reduction program. Int J ObesRelatMetabDisord 25: 1640-1643.
- Nemet D, Oren S, Pantanowitz M, Eliakim A (2013) Effects of a multidisciplinary childhood obesity treatment intervention onadipocytokines, inflammatory and growth mediators.Horm res paediatr 79: 325-332.
- Roth CL, Kratz M, Ralston MM, Reinehr T (2011) Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism 60: 445-452.
- Amati L, Marzulli G, Martulli M, Chiloiro M, Jirillo E (2010) Effects of a hypocaloric diet on obesity biomarkers: prevention of low-gradeinflammation since childhood. Curr pharm des 16: 893-897.
- D’Adamo E, Marcovecchio ML, Giannini C, de Giorgis T, Chiavaroli V, et al. (2013) Improved oxidative stress and cardio-metabolic status in obese prepubertal children with liver steatosis treated with lifestyle combined with Vitamin E. Free Radic Res 47: 146-153.
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 3860
- [From(publication date):
February-2017 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 2938
- PDF downloads : 922