Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Google Scholar citation report
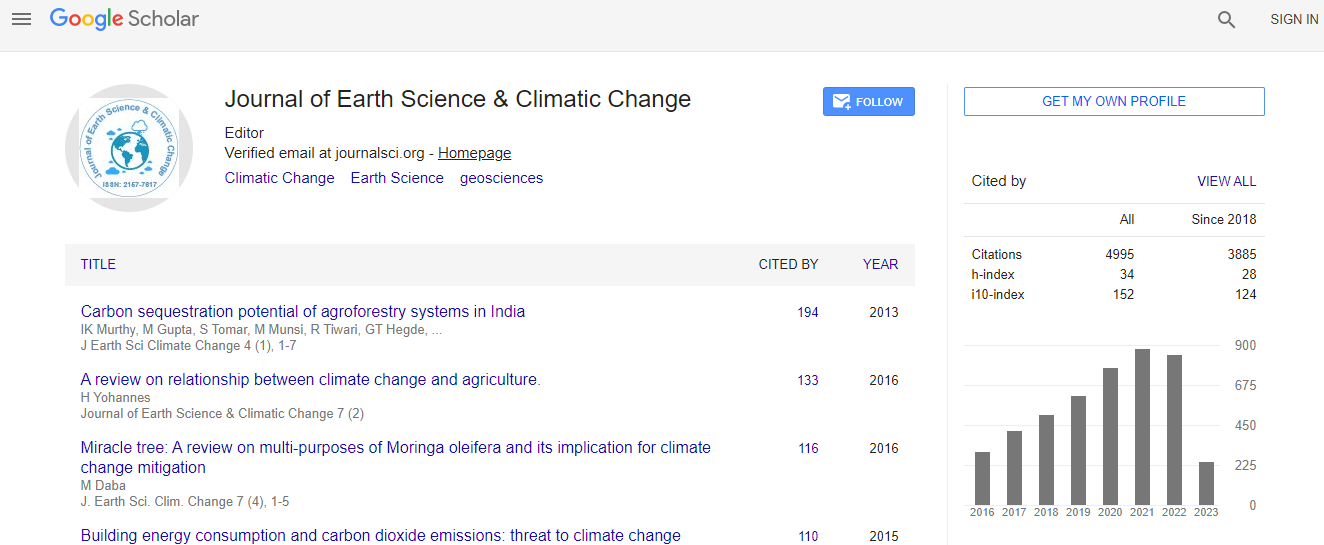
Citations : 5125
Journal of Earth Science & Climatic Change received 5125 citations as per Google Scholar report
Journal of Earth Science & Climatic Change peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- SWB online catalog
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
In Association with

Mineralization of CO2 for Carbon Sequestration using Flue Gas Desulphurization Gypsum
6th World Conference on Climate Change
Jonathan Riddle and Rona J. Donahoe
The University of Alabama, USA
ScientificTracks Abstracts: J Earth Sci Clim Change
Abstract
Past efforts for carbon sequestration using CO2 mineralization have proven effective for long-term stable storage of CO2 but were prohibitive due to slow reaction rates and high cost. Recent mineralization studies reacting flue gas desulphurization (FGD) gypsum (CaSO4â¢2H2O) with CO2 in an alkaline solution, have shown conversion of gypsum into calcium carbonate (CaCO3) to be a rapid and effective method for carbon sequestration. However, these studies used sodium hydroxide and ammonia to increase solution pH. The use of ammonia makes this mineralization method inefficient due to the production of ammonia being a significant CO2 source. The goal of this study was to obtain high FGD gypsum-to-calcite conversation percentages at ambient temperature while eliminating the need for ammonia. A stirred reactor was utilized to study the effects of PCO2 (10, 30, 60, and 250 psi), solution pH (12, 13, 13.5 and 14), solid-to-solution ratio (1:100, 1:80, 1:40, 1:100), and reaction time (10, 15, 30, and 120+ minutes) variation on the rate of conversion. The amount of carbonate produced was calculated from Rietveld refinement of XRD patterns to determine the impact of each variable. Experimental results showed varying degrees of FGD gypsum to CaCO3 conversion. Solution pH was a primary control on conversion rates, with complete conversion occurring under all conditions at pH 13.5 and 14. Time also played a significant role in conversion rates, from 0% conversion at pH 13 after 360 minutes of reaction time, to approximately 75% conversion at pH 13 after 15 minutes of reaction time. The most cost-effective conversions took place at low PCO2 (10 psi), pH 13 and a reaction time of 15 minutes, with a conversion rate of around 75%. The results of this study demonstrate that FGD gypsum can serve as a viable feedstock for CO2 mineralization, potentially providing an inexpensive method for carbon sequestration.Biography
Jonathan Riddle received a bachelor’s degree in science at the University of North Alabama. After receiving his degree, he took a hiatus of four years to teach English in Japan. Afterwards, he decided to continue his geologic career and is currently a master’s student at the University of Alabama. He began studying environmental issues and was awarded the Outstanding Research Paper from Geosyntec in 2018. He has been working with his professor, Dr. Rona Donahoe, on carbon mineralization. His is currently investigating more effective ways to use flue gas desulphurization gypsum for CO2 sequestration. He will be finishing his master’s degree in December of this year in environmental geochemistry.

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi