Research Article Open Access
Nutritional Markers after Loop Duodenal Switch (SADI-S) for Morbid Obesity: A Technique with Favorable Nutritional Outcome
| Atif Abd-Elatif, Tamer Youssef*, Mokhtar Farid, Yasser Ali and Walid Gado | |
| Mansoura Faculty of Medicine, Mansoura University, Mansoura, Egypt | |
| Corresponding Author : | Tamer Youssef Assistant Professor of General and Endocrine Surgery Mansoura University Hospital, Mansoura, Egypt Tel: 002/01223926974 Fax: 002/ 050/ 226701 E-mail: tamyousif@yahoo.com |
| Received April 07, 2015; Accepted June 9, 2015; Published June 30, 2015 | |
| Citation: Abd-Elatif A, Youssef T, Farid M, Ali Y, Gado W (2015) Nutritional Markers after Loop Duodenal Switch (SADI-S) for Morbid Obesity: A Technique with Favorable Nutritional Outcome. J Obes Weight Loss Ther 5:268. doi:10.4172/2165-7904.1000268 | |
| Copyright: © 2015 Abd-Elatif A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Background: A reduction of body weight can be achieved after Biliopancreatic diversion, but there is a risk of malnutrition and diarrhea. This risk may be reduced by pyloric preservation with duodenalswitch. Loop duodenal switch (Single anastomosis duodeno-ileal bypass with sleeve gastrectomy=SADI-S) is hybrid operation combining moderate intake restriction with moderate malabsorption for treatment of morbid obesity. It is considered a modified version of the original duodenal switch operation in which after the sleeve gastrectomy, the duodenum is anastomosed in end to side, ante colic and isoperistaltic manner to the selected ileal loop with a length of 2 meters from ileocacal valve. Objective: To evaluate the nutritional outcomes as well as to determine weight loss success of Loop duodenal switch Procedure as surgical treatment for morbid obesity on a series of 37 consecutively operated patients in Endocrine surgery Unit, Mansoura University hospital, Mansoura University, Mansoura, Egypt. Patients and methods: A prospective study conducted during the period from July 2010 to January 2013. The mean age was 35.37 ± 7.78years. The mean BMI was 56.25 ± 8.43 kg/m². All patients were subjected to Loop Duodenal Switch after preoperative preparation and laboratory investigations including: Haemoglobin, serum iron, serum ferritin, serum vitamin B12, serum folic acid, serum albumen, serum calcium, serum magnesium, serum phosphorus, serum alkaline phosphatase, serum cupper, serum zinc, serum sodium, serum potassium, serum albumen, Aspartate Aminotransferase (AST), Alanine Transaminase (ALT) and serum bilirubin were followed up over 1 year. Results: Most of the patients had smooth postoperative course with no major morbidity and single mortality. The BMI decreased significantly, from: 56.52 ± 8.47, to 33.21 ± 3.91, with decrease of the amount of food ingested. Both hemoglobin and calcium in Loop DS readilyreturned to within the reference range followingsupplementation with iron and calcium respectively. The mean serum iron,serum ferritin, serum vitamin B12, serum folic acid, , serum calcium, serum magnesium, serum phosphorus,serum Alkaline phosphatase,serum cupper, serum zinc, serum sodium, serum potassium, serum albumen, Aspartate Aminotransferase (AST), Alanine Transaminase (ALT) and serum bilirubinremained within the normal range with no significant nutritional deficiency. Conclusion: LoopDS is not associated with broad nutritional deficiencies and does not appear to pose a threat to nutritional status. It provides excellent weight loss with preservation of good alimentation, even in the super obese. Postoperative supplementation with iron, multivitamins, calcium and vitamin D may be required continuously to prevent nutritional deficiency especially for adults and females in the Child bearing period.
Objective: To evaluate the nutritional outcomes as well as to determine weight loss success of Loop duodenal switch Procedure as surgical treatment for morbid obesity on a series of 37 consecutively operated patients in Endocrine surgery Unit, Mansoura University hospital, Mansoura University, Mansoura, Egypt.
Patients and methods: A prospective study conducted during the period from July 2010 to January 2013. The mean age was 35.37 ± 7.78years. The mean BMI was 56.25 ± 8.43 kg/m². All patients were subjected to Loop Duodenal Switch after preoperative preparation and laboratory investigations including: Haemoglobin, serum iron, serum ferritin, serum vitamin B12, serum folic acid, serum albumen, serum calcium, serum magnesium, serum phosphorus, serum alkaline phosphatase, serum cupper, serum zinc, serum sodium, serum potassium, serum albumen, Aspartate Aminotransferase (AST), Alanine Transaminase (ALT) and serum bilirubin were followed up over 1 year.
Results: Most of the patients had smooth postoperative course with no major morbidity and single mortality. The BMI decreased significantly, from: 56.52 ± 8.47, to 33.21 ± 3.91, with decrease of the amount of food ingested. Both hemoglobin and calcium in Loop DS readilyreturned to within the reference range followingsupplementation with iron and calcium respectively. The mean serum iron,serum ferritin, serum vitamin B12, serum folic acid, , serum calcium, serum magnesium, serum phosphorus,serum Alkaline phosphatase,serum cupper, serum zinc, serum sodium, serum potassium, serum albumen, Aspartate Aminotransferase (AST), Alanine Transaminase (ALT) and serum bilirubinremained within the normal range with no significant nutritional deficiency.
Conclusion: LoopDS is not associated with broad nutritional deficiencies and does not appear to pose a threat to nutritional status. It provides excellent weight loss with preservation of good alimentation, even in the super obese. Postoperative supplementation with iron, multivitamins, calcium and vitamin D may be required continuously to prevent nutritional deficiency especially for adults and females in the Child bearing period.
The etiology of morbid obesity is multifactorial and is related to inheritance, physiological, metabolic, sociocultural, behavioral, and psychological factors [2].
People are considered to have morbid obesity if they have a body mass index (BMI) of 40 kg/m2 or more or they have a BMI of between 35 kg/m2 and 40 kg/m2 and other significant disease (for example, diabetes, high blood pressure) that may be improved if they lose weight [3].
Overweight and obesity are estimated to be present in1.7 billion people worldwide [4]. In the Eastern Mediterranean region, the highest levels of overweight persons (BMI ≥ 25) were in Kuwait, Egypt, United Arab Emirates, Saudi Arabia, Jordan and Bahrain, where the incidence of overweight/obesity for those aged ≥ 25 years was between 74%-86% (women) and 69%-77% (men) [5].
Significant comorbidities, defined as medical problems associated with or caused by obesity, are numerous. The most prevalent and acknowledged of these include degenerative joint disease, low back pain, hypertension, obstructive sleep apnea, Gastroesophageal Reflux Disease (GERD), cholelithiasis, type 2 diabetes, hyperlipidemia, hypercholesterolemia, asthma, hypoventilation syndrome of obesity, right-sided heart failure, migraine headaches, pseudotumorcerebri, venous stasis ulcers, deep vein thrombosis, fungal skin rashes, skin abscesses, stress urinary incontinence, infertility, dysmenorrhea, depression, abdominal wall hernias, and an increased incidence of various cancers such as those of the uterus, breast, colon, and prostate [6].
According to the 1991 National Institutes of Health (NIH) consensus conference on gastrointestinal surgery for severe obesity, patients are candidates for bariatric surgery if they are morbidly obese (BMI>40 kg/m2 or ≥ 35 kg/m2 with co-morbidities), have failed attempts at diet and exercise, are motivated and well informed, and are free of significant psychological disease [7].
None of the medical methods of weight reduction provide a lasting weight reduction. Surgery offers the only achievable long-term solution [8]. Bariatric surgery has demonstrated its efficacy in weight loss and in reducing the comorbidities in the morbid obesity patient [9].
Bariatric surgical techniques can be divided into restrictive, malabsorptive, and combined (restrictive and malabsorptive) procedures. Commonly performed procedures include: Laparoscopic Adjustable Gastric Banding (LAGB), Sleeve Gastrectomy (SG), Vertical Banded Gastroplasty (VBG), Roux-en-Y Gastric Bypass (RYGB), Biliopancreatic Diversion (BPD), and BPD with Duodenal Switch (DS) [10].
The biliopancreatic diversion with duodenal switch (BPD-DS) is often referred to as the duodenal switch operation and is a modification of the original biliopancreatic diversion described by Scopinaro in 1979 [11].
The essential difference between these two operations is that in the BPD-DS version, a sleeve gastrectomy is performed and the pylorus is preserved, whereas in the original Scopinaro operation, a distal gastrectomy sacrifices the pylorus. In both operations, the stomach pouch has a capacity of 250 ml and malabsorption results from a distal Roux-en-Y reconstruction of the bowel, with a common channel of 50 to 100 cm and an alimentary limb of 250 cm [12,13].
Proximal duodenal-ileal end-to-side bypass, or loop duodenal switch, is a modification of the original biliopancreatic diversion with duodenal switch [14] in which after the sleeve gastrectomy, the duodenum is anastomosed to the selected ileal loop in a Billroth-II fashion, with no Roux-en-Y reconstruction [15].
SADI-S which is simplified 1-loop duodenal switch with a 200-250 common channel that was introduced by Sánchez-Pernaute A et al. [15]. It has theoretical benefits over RYGP which is considered by many authors to be the gold standard bariatric surgery [16]: half number of performed anastomoses, retained pylorus that controls gastric emptying, no dumping syndrome, reduction of operative time and possible lower rate of postoperative complications [15]; But due to the novelty of this procedure the effects on nutritional status have yet to be clearly defined.
The aim of this study was to establish baseline nutritional status, record prevalence of nutritional deficiency, as well as determine weight loss success in the Loop Duodenal Switch patient during the first 12 month post-surgery.
The preoperative evaluation included careful history taking regarding age, sex, obesity associated comorbidities, clinical examination including weight by kg, height by meter and Body Mass Index (BMI), body circumferences, blood pressure and laboratory investigations included: Haemoglobin, serum iron, serum ferritin, serum vitamin B12, serum folic acid, serum magnesium, serum phosphorus, serum alkaline phosphatase, serum cupper, serum zinc, serum sodium, serum potassium, serum albumen, Aspartate Aminotransferase (AST), Alanine Transaminase (ALT) and serum bilirubin. In addition to the routine preoperative assessment as for any other major abdominal surgery, the patient may undergo further assessment for pulmonary functions, endocrine disorders or gastro-esophageal disorders.
Patients were advised to lose weight prior to surgery to help to facilitate the operative procedure by placing them on protein diet for 2 weeks prior to surgery. This helps to shrink the visceral fat and particularly the fatty deposits within the liver. Patients were admitted to the hospital one day before surgery, at which time they underwent most of their preoperative tests. Upon admission, they were seen by an anaesthesiologist and an internist, and received bowel preparation. Patients were given Enoxaparin (Clexan) 60 mg on the evening of admission, and daily thereafter during the hospital stay. An epidural catheter was placed for postoperative pain management. Sequential compression stockings were used.
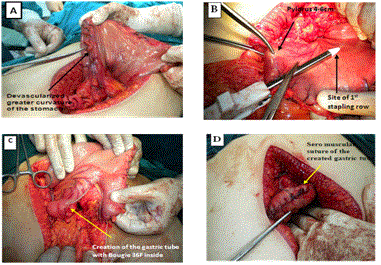
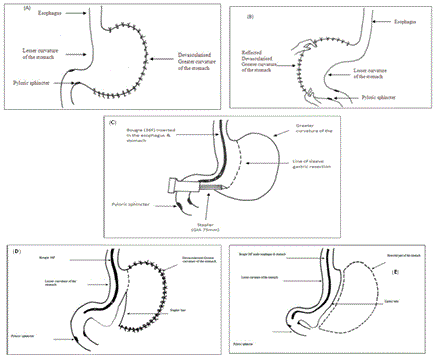
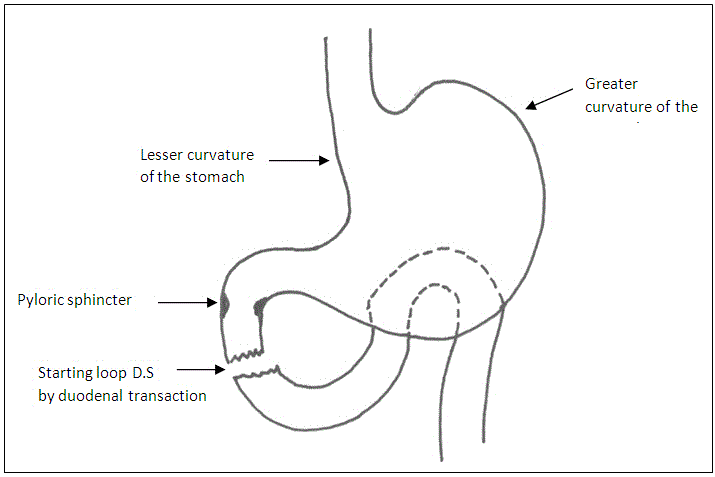
The sleeve gastorectomy was performed by mobilizing the greater curvature of the stomach distally to about 4 cm past the pylorus and proximally to the angle of His; using ultrasonic shears .The stomach and fundus were fully mobilized during the dissection. The filmy posterior attachments were divided so the entire posterior surface of the stomach can be seen. Once this dissection was complete, the first stapler was placed tangentially across the antrum. The authors used green loads for the first two staple firings because of the increased thickness of the stomach in this area. The assistant flattened the stomach with lateral retraction and the anesthesiologist removed the orogastric tube before the first staple firing. The angle of the first firing was determined by the patient’s anatomy, but care should be taken to not use an angle that will narrow the lumen at the incisura. Next 36 Fr. Bougie was passed into the stomach along the lesser curve to the pylorus. If any concern existed that the lumen is too narrow at the incisura, the stapler was moved laterally before firing. Once the surgeon was satisfied with the lumen size, the stapler was fired. Care was taken to create a straight staple line and avoid anterior or posterior “spiraling” of the staple line. The position of the final staple firing was critical to avoid a leak by avoiding the gastro esophageal junction and esophagus during firing. Approximately 1cm of gastric serosa should be seen to the left of the stapler cartridge before the stapler is fired. The entire staple line is then oversewn with continuous, imbricating Vicryl 2/0 sutures (Figure 1 and Diagram 1).
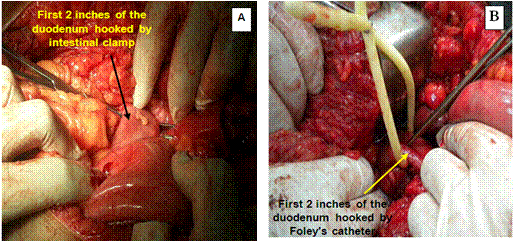
The duodenum was sectioned at the level of the gastroduodenal artery, warranting a 2-4 cm proximal duodenal stump and avoiding damage to the common bile duct (Figure 2 and Diagram 2).
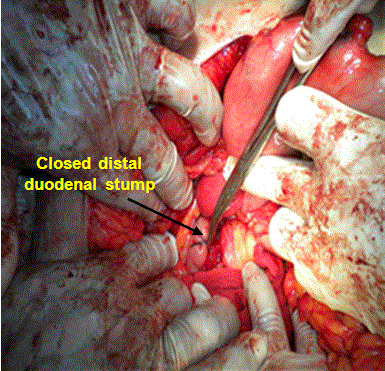
The distal duodenal stump was over sutured in 2 layers; through and through layer using Vicryl 2/0 sutures, seromuscular layer using silk 2/0 sutures (Figure 3 and Diagram 3).
We observed that starting the Loop D.S technique with duodenal transection facilitates the sleeve gastric resection.
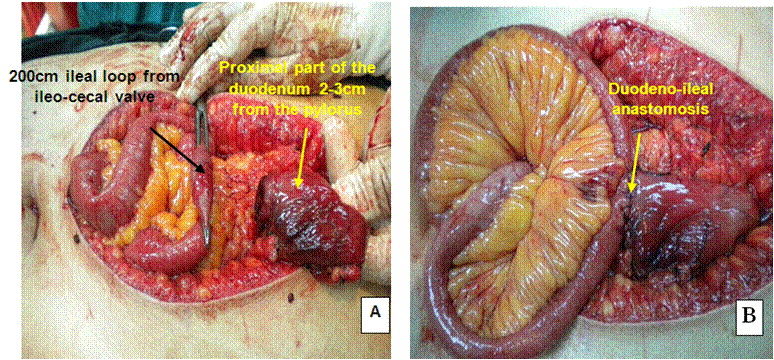
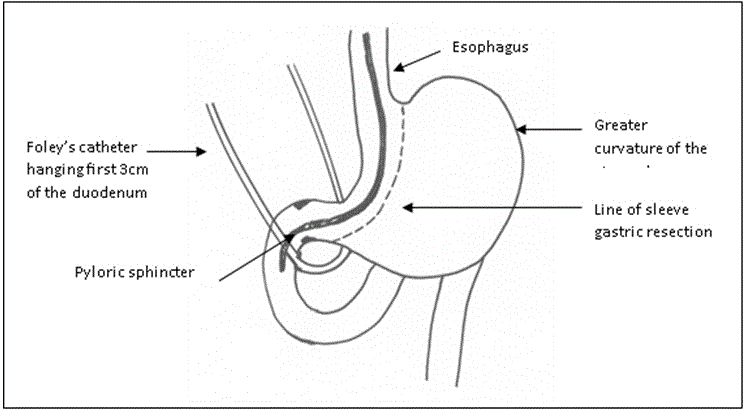
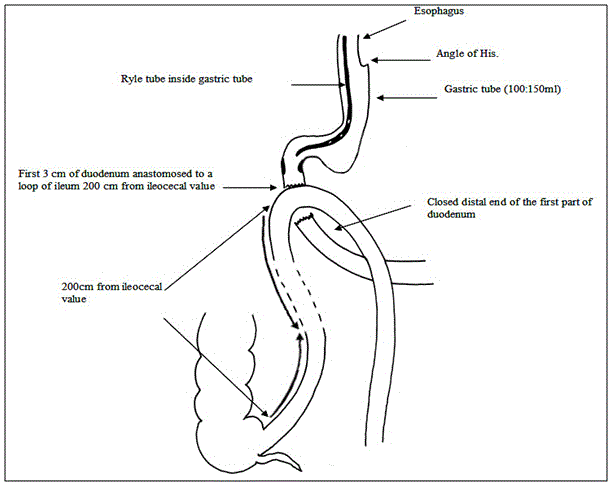
The ileo-cecal junction was identified, and 250 cm was measured upwards. The selected loop was ascended up and anastomosed to the proximal duodenal stump in an isoperistaltic way end-to-side hand-sewn anastomosis (Figure 4 and Diagram 4).
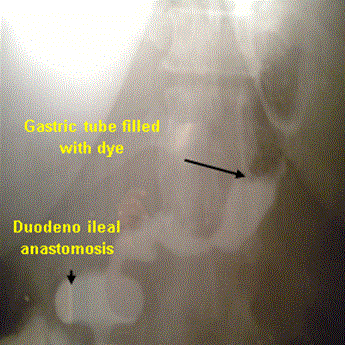
ative course included: Proton pump inhibitor was administrated routinely in all patients. Ryle tube was removed at the 2nd postoperative day. Gastrografin meal was performed at the 5th postoperative day (Figure 5) and oral fluids intake was started if there was no leak. Early ambulation was advised from the first postoperative day. Drain was removed once oral intake was started after assurance that was no leak. The patients were discharged 6-9 days postoperatively according to postoperative course.
After discharge patients continued on clear fluids for one week (sugar free) then full fluids during the second week and soft food was allowed on the third week followed by regular food. Supplementation with Calcium, Vitamin D, Vitamin B12, Iron and multivitamins was done.
Follow up visits were scheduled postoperatively at three weeks, three months and then every 3 months. Follow up included clinical examination to detect any postoperative complications, body weight and BMI, laboratory investigations included: CBC, iron, ferritin, vitamin B12, folic acid, serum sodium and potassium, calcium, phosphorus, magnesium, alkaline phosphatase, serum albumen and liver function tests were performed at each visit.
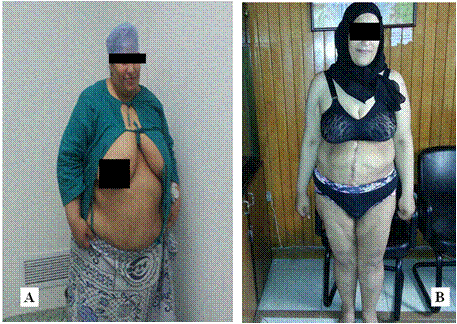
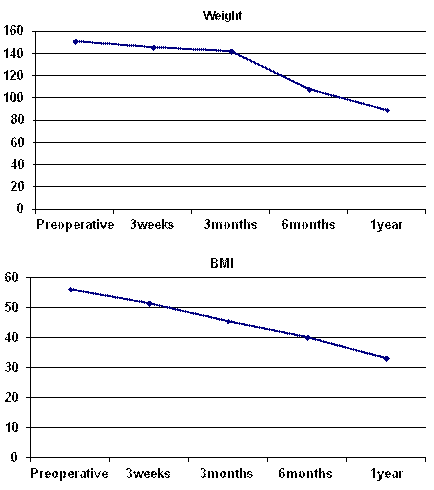
There is a highly statistical significant difference between mean scores of weight and BMI before surgery and after 3, 6 and 12 months after surgery (Table 2 and Diagram 5). The mean weight of our patients decreased significantly from 150.75 ± 20.15 kg preoperatively to 89.13 ± 10.09 kg one year after surgery (p<0.001) with a mean reduction in weight of 61.62 kg. The mean BMI decreased significantly from 56.25 ± 8.43 kg/m2 preoperatively to 33.21 ± 3.91 kg/m2 one year after surgery (p=0.001) At 3 weeks, 3 months, 6 months and one-year follow up, the mean reduction in BMI was 4.49 kg/m2, 10.67 kg/m2, 16.03 kg/m2 and 23.04 kg/m2 respectively.
As regard Postoperative Nutritional and laboratory follow up (Table 3): All patients receive follow-up nutritional counselling for a protein-enriched diet (80 to 100 g/day), and multivitamins, iron, and fat-soluble vitamins (D, E, A, and K) are given on a daily basis. The mean level of haemoglobin shows an increase from 10.85 ± 0.68 mg/dl preoperative to 11.47 ± 0.59 mg/dl at the end of the first year (not significant); also the mean haematocrit value shows an increase from 34.45 ± 2.83 mg/dl preoperative to 35.31 ± 1.85 mg/dl at the end of the first year (not significant). The mean serum Iron increased from 87.19 ± 28.65mg/dl preoperative to 97.69 ± 16.67 mg/dl at the end of the first year. Averages for each time-point fell within normal reference ranges (not significant). The mean serum Ferritin increased from 105.89±47.10mg/dl preoperative to 123.26 ± 36.21 mg/dl at the end of the first year. Averages for each time-point fell within normal reference ranges (not significant). The mean serum Magnesium decreased from 1.94 ± 0.24 mg/dl preoperative to 1.86 ± 0.27 mg/dl at the end of the first year. Averages for each time-point fell within normal reference ranges (not significant).
The mean serum Zinc increased from 80.32 ± 11.48 mg/dl preoperative to 83.58 ± 11.59 mg/dl at the end of the first year. Averages for each time-point fell within normal reference ranges (not significant). The mean serum Cupper increased from99.72 ± 19.33 mg/dl preoperative to 102.60 ± 19.66 mg/dl at the end of the first year. Averages for each time-point fell within normal reference ranges (not significant). The mean serum Vitamin B12 increased from 479.08 ± 153.70 mg/dl preoperative to 520.82 ± 169.60 mg/dl at the end of the first year. Averages for each time-point fell within normal reference ranges (not significant). The mean serum Folic acid increased from 10.48 ± 4.07 mg/dl preoperative to 12.01 ± 2.44 mg/dl at the end of the first year. Averages for each time-point fell within normal reference ranges (not significant). The average values of serum electrolytes for each time-point fell within normal reference ranges (not significant).
After surgery, all patients received oral supplementation of 1000 mg calcium and 800 IU vitamin D per day. From this table, it is shown that the mean serum calcium decreased from 8.82 ± 0.29 mg/dl preoperative to 8.80 ± 0.29 mg/dl at the end of the first year (not significant); the mean serum Phosphorus decreased from 3.81 ± 0.56 mg/dl preoperative to 3.81 ± 0.28 mg/dl at the end of the first year. Averages for each time points fell within normal reference ranges (not significant); also the mean serum.
Parathyroid Hormone (PTH) levels increased significantly from a preoperative level of 21.79 ± 4.69 pg/ml to 70.39 ± 21.12 pg/ml at the end of the first year but averages for each time-point fell within normal reference ranges. Lastly, the mean value of Bone Specific Alkaline Phosphatase (BSAP) levels increased significantly from a preoperative level of 59.72 ± 15.19 ng/ml to 74.65 ± 15.44 ng/ml at the end of the first year. But averages for each time-point fell within normal reference ranges (Figure 6 and 7).
The mean serum Albumen shows a significant decrease from 4.00 ± 0.19 mg/dl preoperatively to 3.70 ± 0.22 mg/dL at the end of the first year but still within the normal reference range. The mean serum SGOT, SGPT and sr. bilirubin show no significant changes from the preoperative values.
In the previous literature by Sánchez-Pernaute A et al. [15] sleeve gastrectomy was done before duodenal switch transection. In our study, duodenal transection was done primarily in most of the cases (Abdlatif Modification). We observed that starting with duodenal transection facilitates the later sleeve gastric resection.
In our study weight loss was assessed by decrease in BMI one year after surgery. We achieved a decrease in BMI from 56.25 ± 8.43 kg/m2 to 33.21 ± 3.91 kg/m2. This reduction in BMI was higher than that reported after BPD [18]; as they reported that the decrease in BMI was from 47.46 ± 9.5 to 30.86 ± 5.6. It was also pointed that DS patients decreased their BMI from a mean of 55.2 kg/m2 to 32.5 kg/m2 [19]; whereas the RYGB patients decreased their BMI from 54.8 kg/m2 to 38.5 kg/m2. These results are comparable to our results.
Many obese persons undergoing WLS have preoperative nutritional deficiencies [20] that can be exacerbated by malabsorptive procedures. Even patients undergoing purely restrictive procedures are at risk for nutritional deficiencies due to poor eating habits as well as food intolerances and eating restrictions [21,22].
We agree that all malabsorptive procedures as duodenal switch usually require strict nutritional supplementation especially postoperatively as most of the Intestinal tract is bypassed. This heightened risk underscores the importance of lifelong follow-up of WLS patients, and the need for clinicians to have a high index of suspicion for nutritional-related abnormalities. Patients may suffer malnutrition due to incompliance and/or operation complications [23].
Although multivitamins typically contain the U.S. Recommended Daily Allowance for most vitamins and minerals such as iron and calcium, the available data show that multivitamins alone do not consistently protect patients from metabolic deficiencies after either RYGB or BPD. The one exception is folate, which can be maintained at normal levels in patients who regularly take multivitamins after RYGB [24,25].
In our series, after loopduodenal switch procedure, the degree of anemia usually was mild; we reported average mean values of both iron and ferritin and gradual improved values of both hemoglobin and hematocrit towards the end of the first year with patient compliance and regular follow up. On the other hand, a certain percentage of patients usually develop iron and ferritin deficiency after any operation which bypasses the duodenum, as RYGB while iron deficiency did not differ in BPD with or without duodenal switch, and proved to occur in some studies [26,27] while others not [28]. This is because iron is absorbed preferentially in the distal duodenum and proximal jejunum. The frequency of anemia after duodenal switch (about 50%) is comparable to that after RYGB, where it varied from 41% after a short limb to 74% after a long Roux limb [29].
Vitamin B12 and folate deficiencies are often evaluated together. Studies indicate that these deficiencies are fairly prevalent after bariatric operations despite the patients being advised to follow a multivitamin regimen [26,29,30]. After 1year of RYGB follow up Vitamin B12 and folic acid deficiency were reported to be 33%, 63%respectively [31] and that value was higher than that after BPD (22%) after 4 years of follow up [26]. We reported only 5.4% and 2.7 % of Vitamin B12 and folic acid deficiencies during our follow up period especially with good supplementation and patient compliance; although that, the mean value did not alter significantly over 1 year. We agree that vitamin B12 and folic acid deficiency does not usually occur in patients after the duodenal switch in contrast to that was reported after Roux-en-Y gastric bypass due to the preservation of more gastric mucosa that secreted inadequate intrinsic factor may not lead to a vitamin B-12 deficiency with the duodenal switch procedure [26,32].
We also did not report Zinc deficiency. Although it is seen mainly after BPD, [33] but can also occur after purely restrictive procedures due to poor dietary intake [34].
Parathyroid hormone (PTH) level showed slightly elevated levels but fluctuating within normal reference range, it goes ahead with other studies conducted [35]. Serum calcium and phosphorus in our study showed levels move within normal reference range, but this with continues supplementation it goes ahead with other studies conducted [35]. In comparison, Dolan et al. [27] compared the nutritional side-effects of BPD alone vs BPD-DS, by performing a nutritional screen at a median follow-up of 28 months in both groups of patients. One-quarter of the patients were hypocalcaemia despite more than 80% taking vitamin supplements. There were no significant differences between the BPD and BPD-DS, suggesting that duodenal switch does not lessen the nutritional side-effects of BPD [27].
Bone specific alkaline phosphatase (BSAP) (marker of bone formation) showed levels fluctuating within normal reference range in most patients of our study.
Although there is no reported evidence of clinical complications of magnesium deficiency following bariatric surgery [36], we did not report magnesium deficiency in our series. Marceau et al. [37] found also no significant abnormalities in magnesium levels before BPD, after 4 years and after 10 years.
According to Scopinaro N et al. [38] in all BPD patients, protein malnutrition is a major concern. It is manifested by clinical hypoalbuminemia, edema, asthenia, and alopecia. In our study, only one (2.7%) patient developed protein malnutrition. We treated him conservatively by continuous 3 weeks of parenteral feeding and he showed good improvement then he continued on high protein diet and protein rich formulas. After loop DS also reported 4(8%) cases of significant protein malnutrition, 2 of whom underwent revision to RYGB. This rate considered to be high that made him later change the common limb length to from 200 to 250 cm [35]. After open BPD, it was reported that 91(6.7%) of cases developed protein malnutrition [38].
Our data was consistent with that of Sánchez-Pernaute A et al. [35] that there was no evidence of hepatic dysfunction or liver failure manifested by either elevated serum transaminases or bilirubin levels.
In our series, the average number of bowel movements was 2.5/day, which was similar to that reported after loop DS [35]. We did not record any cases of excessive diarrhea which is frequent episodes of diarrhea not adequately controlled. After open classical DS, 2(0.45%) cases of excessive diarrhea that required revision surgery to lengthen both the common and alimentary channels [39].
References
- Deitel M, Shikora SA (2002) The development of the surgical treatment of morbid obesity. J Am Coll Nutr 21: 365-371.
- Li Z, Bowerman S, Heber D (2005) Health ramifications of the obesity epidemic. Surg Clin North Am 85: 681-70, v.
- Santos LM, de Oliveira IV, Peters LR, Conde WL (2010) Trends in morbid obesity and in bariatric surgeries covered by the Brazilian public health system. Obes Surg 20: 943-948.
- Deitel M (2003) Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg 13: 329-330.
- El Ansari W, El Ashker S, Moseley L (2010) Associations between physical activity and health parameters in adolescent pupils in Egypt. Int J Environ Res Public Health 7: 1649-1669.
- Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB (2003) Years of life lost due to obesity. JAMA 289: 187-193.
- National Institutes of Health (NIH) (1991) Gastrointestinal Surgery for Severe Obesity. Consensus Statement 25-27 9: 1-20. And NIH conference Consensus Development Conference Panel (1991): Gastrointestinal surgery for severe obesity. Ann Intern Med 1991 115: 956-961,
- Agaba EA, Shamseddeen H, Gentles CV, Sasthakonar V, Gellman L, et al. (2008) Laparoscopic vs open gastric bypass in the management of morbid obesity: a 7-year retrospective study of 1,364 patients from a single center. Obes Surg 18: 1359-1363.
- Cánovas B, Sastre J, Neblett A, López-Pardo R, Abad S, et al. (2006) [Techniques of bariatric surgery: analysis of 78 cases]. Nutr Hosp 21: 567-572.
- Korenkov M (2006) Bariatric surgery. Contrib Nephrol 151: 243-253.
- Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V (1979) Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg 66: 618-620.
- Marceau P, Biron S, Bourque RA, Potvin M, Hould FS, et al. (1993) Biliopancreatic Diversion with a New Type of Gastrectomy. Obes Surg 3: 29-35.
- Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, et al. (1998) Biliopancreatic diversion with duodenal switch. World J Surg 22: 947-954.
- Hess DS, Hess DW (1998) Biliopancreatic diversion with a duodenal switch. Obes Surg 8: 267-282.
- Sánchez-Pernaute A, Rubio Herrera MA, Pérez-Aguirre E, García Pérez JC, Cabrerizo L, et al. (2007) Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg 17: 1614-1618.
- Cottam DR, Fisher B, Sridhar V, Atkinson J, Dallal R (2009) The effect of stoma size on weight loss after laparoscopic gastric bypass surgery: results of a blinded randomized controlled trial. Obes Surg 19: 13-17.
- van den Heuvel M, Hörchner R, Wijtsma A, Bourhim N, Willemsen D, et al. (2011) Sweet eating: a definition and the development of the Dutch Sweet Eating Questionnaire. Obes Surg 21: 714-721.
- Scopinaro N, Adami GF, Marinari GM, Gianetta E, Traverso E, et al. (1998) Biliopancreatic diversion. World J Surg 22: 936-946.
- Søvik TT, Aasheim ET, Taha O, Engström M, Fagerland MW, et al. (2011) Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann Intern Med 155: 281-291.
- Alvarez-Leite JI1 (2004) Nutrient deficiencies secondary to bariatric surgery. Curr Opin Clin Nutr Metab Care 7: 569-575.
- Giusti V, Suter M, Héraïef E, Gaillard RC, Burckhardt P (2004) Effects of laparoscopic gastric banding on body composition, metabolic profile and nutritional status of obese women: 12-months follow-up. Obes Surg 14: 239-245.
- Chapman AE, Kiroff G, Game P, Foster B, O'Brien P, et al. (2004) Laparoscopic adjustable gastric banding in the treatment of obesity: a systematic literature review. Surgery 135: 326-351.
- Aasheim ET, Björkman S, Søvik TT, Engström M, Hanvold SE, et al. (2009) Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr 90: 15-22.
- Updegraff TA, Neufeld NJ (1981) Protein, iron, and folate status of patients prior to and following surgery for morbid obesity. J Am Diet Assoc 78: 135-140.
- Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, et al. (1998) Are vitamin B12 and folate deficiency clinically important after roux-en-Y gastric bypass? J Gastrointest Surg 2: 436-442.
- Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, et al. (2002) Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg 12: 551-558.
- Dolan K, Hatzifotis M, Newbury L, Lowe N, Fielding G (2004) A clinical and nutritional comparison of biliopancreatic diversion with and without duodenal switch. Ann Surg 240: 51-56.
- Rabkin RA, Rabkin JM, Metcalf B, Lazo M, Rossi M, et al. (2004) Nutritional markers following duodenal switch for morbid obesity. Obes Surg 14: 84-90.
- Brolin RE, LaMarca LB, Kenler HA, Cody RP (2002) Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg 6: 195-203.
- Halverson JD (1986) Micronutrient deficiencies after gastric bypass for morbid obesity. Am Surg 52: 594-598.
- Brolin RE, Gorman RC, Milgrim LM, Kenler HA (1991) Multivitamin prophylaxis in prevention of post-gastric bypass vitamin and mineral deficiencies. Int J Obes 15: 661-667.
- Marcuard SP, Sinar DR, Swanson MS, Silverman JF, Levine JS (1989) Absence of luminal intrinsic factor after gastric bypass surgery for morbid obesity. Dig Dis Sci 34: 1238-1242.
- Slater GH, Ren CJ, Siegel N, Williams T, Barr D, et al. (2004) Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 8: 48-55.
- Trostler N, Mann A, Zilberbush N, Charuzi I I, Avinoach E (1995) Nutrient Intake following Vertical Banded Gastroplasty or Gastric Bypass. Obes Surg 5: 403-410.
- Sánchez-Pernaute A, Rubio MÁ, Pérez Aguirre E, Barabash A, Cabrerizo L, et al. (2013) Single-anastomosis duodenoileal bypass with sleeve gastrectomy: metabolic improvement and weight loss in first 100 patients. Surg Obes Relat Dis 9: 731-735.
- Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S (2005) Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg 15: 145-154.
- Marceau P, Biron S, Lebel S, Marceau S, Hould FS, et al. (2002) Does bone change after biliopancreatic diversion? J Gastrointest Surg 6: 690-698.
- Scopinaro N (2006) Biliopancreatic diversion: mechanisms of action and long-term results. Obes Surg 16: 683-689.
- Hess DS, Hess DW (1998) Biliopancreatic diversion with a duodenal switch. Obes Surg 8: 267-282.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
 |
 |
 |
 |
 |
| Diagram 1 | Diagram 2 | Diagram 3 | Diagram 4 | Diagram 5 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 21303
- [From(publication date):
June-2015 - Sep 01, 2025] - Breakdown by view type
- HTML page views : 16474
- PDF downloads : 4829
