Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
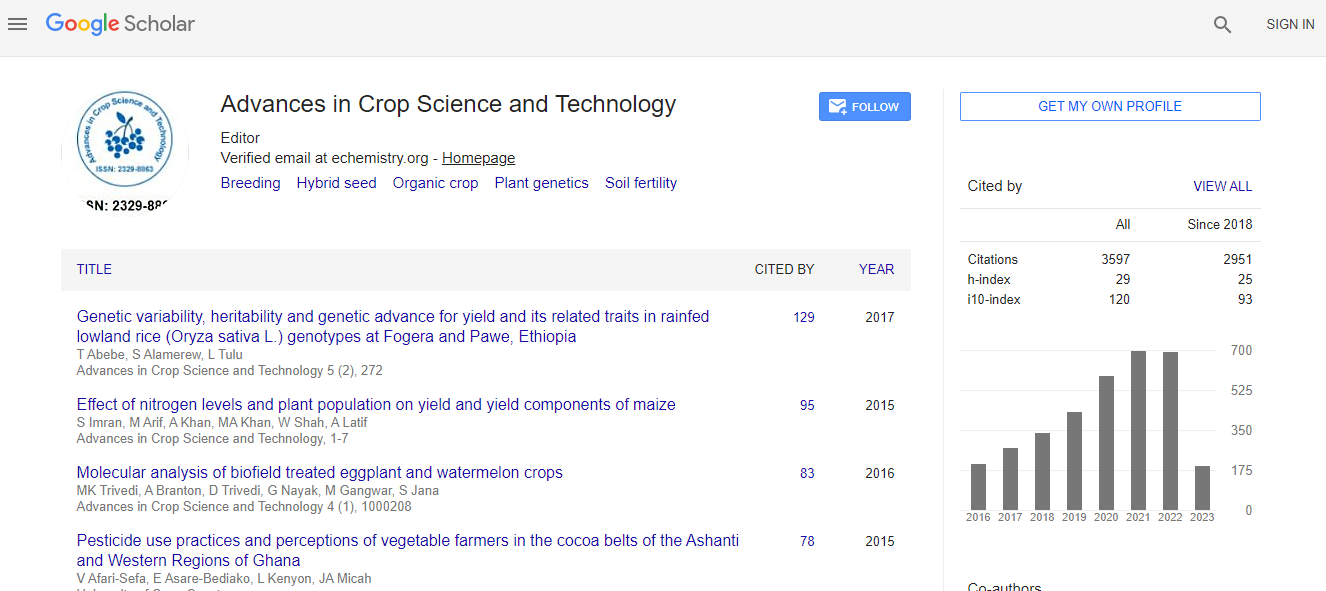
Google Scholar citation report
Citations : 5759
Advances in Crop Science and Technology received 5759 citations as per Google Scholar report
Advances in Crop Science and Technology peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Academic Keys
- JournalTOCs
- Access to Global Online Research in Agriculture (AGORA)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
Useful Links
Recommended Journals
Related Subjects
Share This Page
Enthalpy-entropy compensation analysis of the powder of Taraxacum officinale√ʬ?¬?s leaves in moisture sorption phenomena
11th World Congress on Plant Biotechnology and Agriculture
Moussaoui Haytem, Idlimam Ali and Lamharrar Abdelkader
Cadi Ayyad University, Morocco
ScientificTracks Abstracts: Adv Crop Sci Tech
Abstract
Taraxacum officinale is a kind of flowering plant that belongs to a vast family of Asteraceae. This family is represented by more than 2500 species. They are utilized for various purposes that include for dyspepsia, bile stimulation, bruises, rheumatism, eczema and muscle aches. Moreover, the powder of dandelion is used as special ingredients in soups, salads, green tea and wines. The Taraxacum officinale used in this study were collected locally in Settat region, Morocco. The aim of this study is to determine the experimental sorption isotherms and the net isosteric heat of sorption to conserve the powder. These isotherms are a powerful tool to know the state of water inside the product and his functional availability in the biological and chemical substances. The sorption isotherm curves were determined experimentally for the carob seeds at 3 temperatures (30, 40, 50 √?¬įC) and relative humidity within the range of 5-90% commonly used in the drying and storage. The net isosteric heats of sorption of water were determined from the equilibrium data at different temperatures. The compensation theory was further used to good straight lines were observed for adsorption and desorption. The Gibbs free energy values are positive (√?¬?G√?¬≤>0), indicating that the sorption processes are not spontaneous.Biography
Moussaoui Haytem is currently a PhD student and a Member in Laboratory of Solar Energy & Aromatic and Medicinal Plants, ENS Marrakesh, Physics Department, Cadi Ayyad University, Marrakech, Morocco. He is also an Industrial Engineer. Moreover, he is a Member in Techno-Sharing Club for Student Development. He has participated in several social activities whose aim is to help the homeless children and the old men in house of the elderly.
Email:nabilbouchra23@gmail.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi