Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
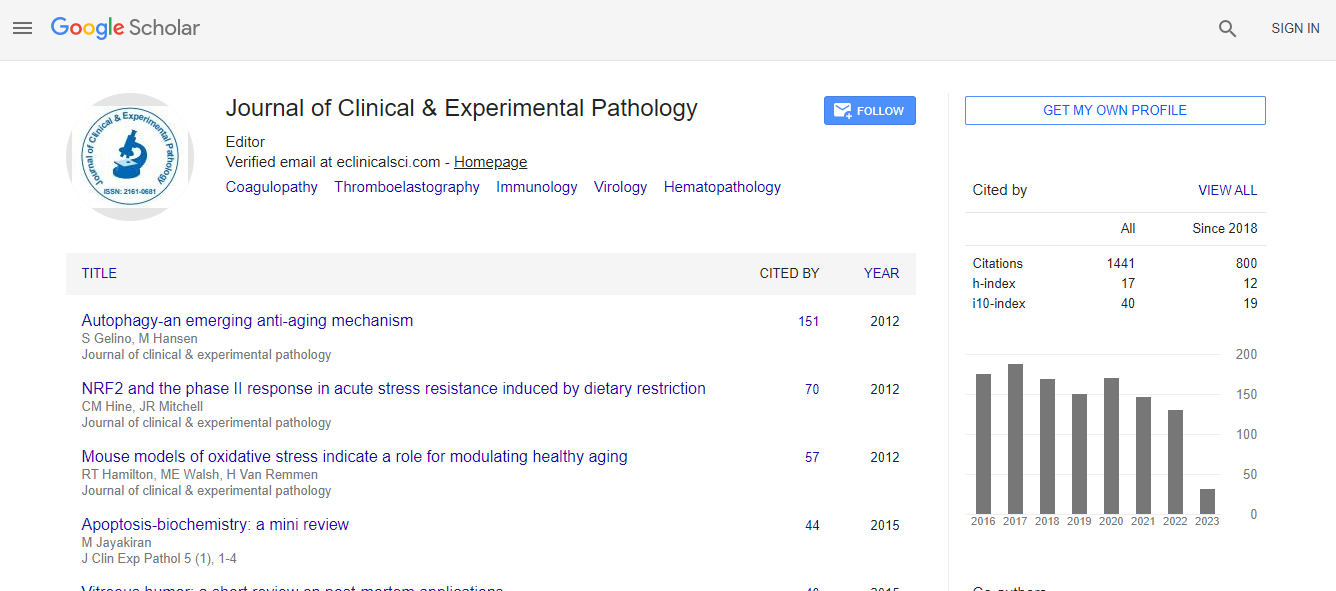
Google Scholar citation report
Citations : 2975
Journal of Clinical & Experimental Pathology received 2975 citations as per Google Scholar report
Journal of Clinical & Experimental Pathology peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Cosmos IF
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
- world cat
- journal seek genamics
- j-gate
- esji (eurasian scientific journal index)
Useful Links
Recommended Journals
Related Subjects
Share This Page
The paris system for reporting urinary cytology: A paradigm shift
5th International Conference on Pathology
Guliz A Barkan
Loyola University Medical Center, USA
Keynote: J Clin Exp Pathol
Abstract
More than fifty years ago, Dr. George Papanicolaou hypothesized that the evaluation of exfoliated cells in urine was a potentially useful method to detect urinary tract malignancies. Since then, urinary cytology has been plagued by less than a stellar literature that showed problems with sensitivity, accuracy and reproducibility. The main purpose of urine cytology is to detect high grade urothelial carcinoma (HGUC). With this principle in mind, The Paris System (TPS) Working Group, composed of cytopathologists, surgical pathologists, and urologists, has proposed and published a standardized reporting system that includes specific diagnostic categories and cytomorphological criteria for the reliable diagnosis of HGUC. This lecture will discuss the outlines, the essential elements of TPS and the process that led to the formation and rationale of the reporting system. The Paris System Working Group, which was organized at the 2013 International Congress of Cytology, conceived a standardized platform on which to base cytologic interpretation of urine specimens. The widespread dissemination of this approach to cytologic examination and reporting of urologic samples and the scheme├ó┬?┬?s universal acceptance by pathologists and urologists is critical for its success. For urologists, understanding the diagnostic criteria, their clinical implications, and limitations of TPS is essential if they are to utilize urine cytology and non-invasive ancillary tests in a thoughtful and practical manner. This is the first international/inclusive attempt at standardizing urinary cytology. The success of TPS will depend on the pathology and urology communities working collectively to improve this seminal paradigm shift, and optimize the impact on patient care.Biography
Güliz A Barkan received her medical degree from Marmara University School of Medicine in Istanbul, Turkey in 1995. She completed residency in Anatomic and Clinical Pathology at The University of Michigan, Ann Arbor, Michigan in 2001. She finished a Surgical Pathology fellowship in the same institution in 2002 and a Cytopathology fellowship at the University of Texas M.D. Anderson Cancer Center, Houston, Texas in 2003. After a brief period in private practice in Turkey she joined Loyola University Department of Pathology in January 2006. She is currently the Director of Cytology, the Director of the Residency Program in Anatomic and Clinical Pathology, the Director of the Cytopathology Fellowship Program and the vice chair of education at Loyola University Medical Center. Dr. Barkan is serving as a member of the American Board of Pathology Test Committee, and a member of the Pathology Residency Program Directors Council. Actively involved with the American Society of Cytopathology, she is a member of the Executive Board and current chair of the Cyto-e conference Committee. She is also a fellow of the International Academy of Cytology.
Email: GBARKAN@lumc.edu

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi