Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
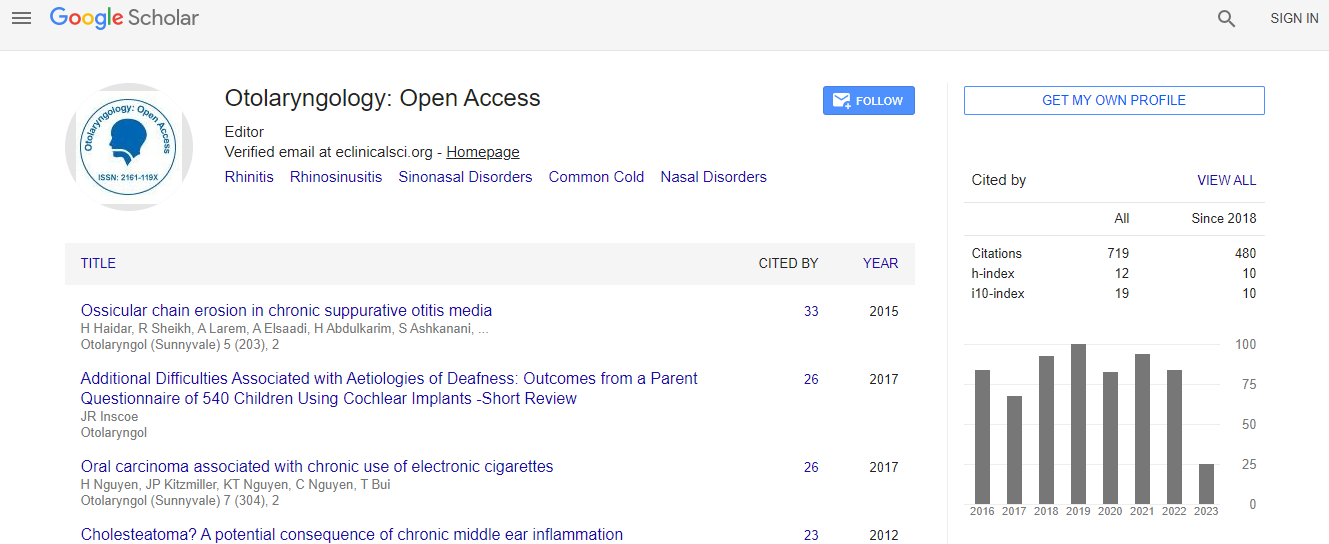
Google Scholar citation report
Citations : 925
Otolaryngology: Open Access received 925 citations as per Google Scholar report
Otolaryngology: Open Access peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Two year results of clinical efficacy of cisplatin in combination with sodium thiosulfate (STS) vs. cisplatin alone in a randomized phase III trial for standard risk hepatoblastoma (SR-HB) SIOPEL 6
International Conference on Aesthetic Medicine and ENT
Kaukab Rajput
Great Ormond Street Hospital, UK
Posters & Accepted Abstracts: Otolaryngology
Abstract
Background: A serious permanent side effect of cisplatin (Cis) therapy is bilateral high-frequency hearing loss which is particularly debilitating when it occurs at a young age. Sodium thiosulfate (STS) has been shown to dramatically reduce hearing loss in children treated with cisplatin containing chemotherapy without tumor protection in localized disease. Methods: Newly diagnosed SR-HB patients were randomized to Cis or Cis+STS for 4 preop and 2 postop courses. Cis 80mg/m2 was administered over 6 hrs. STS was administered exactly 6 hours after stop Cis over 15 minutes at 20 g/m2. Tumor response was assessed after 2 and 4 cycles preop with serum AFP and liver imaging. In case of progressive disease STS was to be stopped and chemotherapy changed to combination therapy with Cis and doxorubicin 60mg/m2. The primary endpoint of the trial is centrally reviewed absolute hearing threshold, at the age of �3.5 years, by pure tone audiometry. Secondary endpoints are event free (EFS) and overall survival (OS). Results: 109 patients (52 Cis and 57 Cis+STS) were recruited at trial closure in December 2014. The combination of Cis+STS was generally well tolerated. The median follow up is 32 months and provisional 2 years EFS is Cis 86.3% and Cis+STS 89.0%; 2 years OS is Cis 91.4% and Cis+STS 97.7%. Treatment failure defined as PD at 4 cycles was equivalent in both arms (3 Cis; 3 Cis+STS). As of February 2016, 5 patients had died (4 Cis; 1 Cis+STS), 1 had relapsed (Cis+STS) and 1 was still in PR (Cis+STS). Interim results of centrally reviewed and Brock graded audiograms for 68 patients at age >3.5yrs are encouraging. Definitive results will become available end 2017. Conclusion: This randomized phase III trial in standard risk hepatoblastoma of cisplatin alone vs. cisplatin plus the otoprotectant STS shows comparable 2 years EFS and OS with no evidence of tumor protection.Biography
Email: Kaukab.Rajput@gosh.nhs.uk

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi