Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
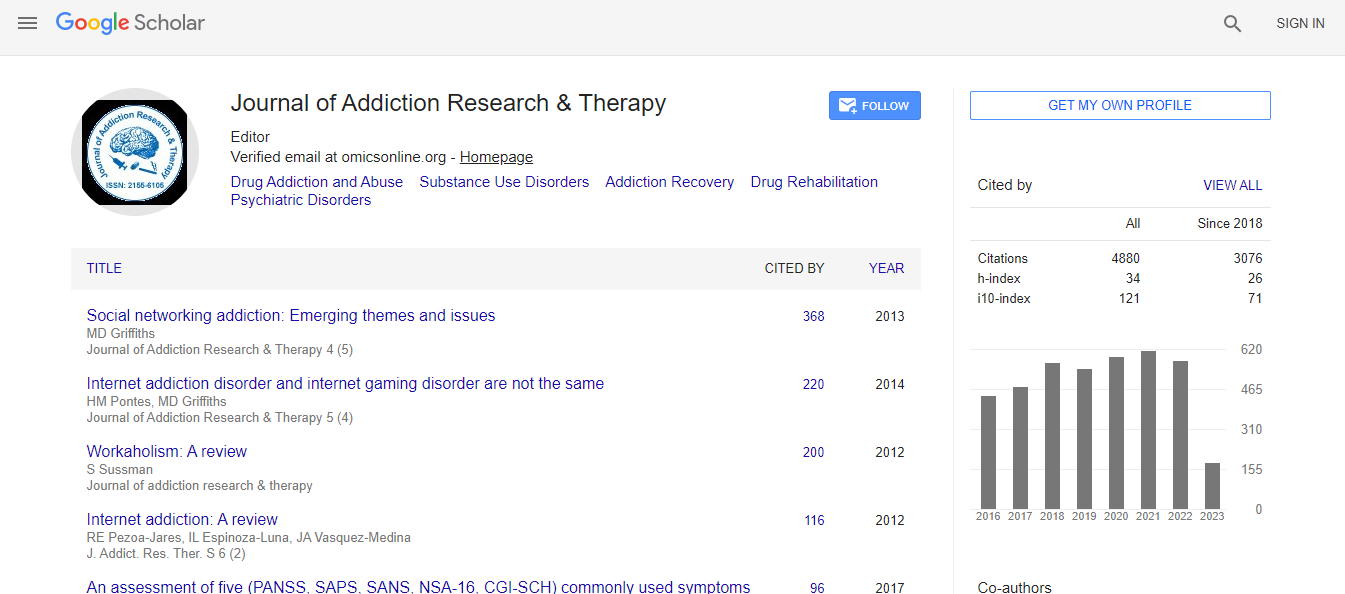
Google Scholar citation report
Citations : 4859
Journal of Addiction Research & Therapy received 4859 citations as per Google Scholar report
Journal of Addiction Research & Therapy peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- SafetyLit
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Factors associated with non-adherence to buprenorphinenaloxone among opioid dependent African-Americans: a retrospective chart review
8th International Conference on Addiction Psychiatry
Suneeta Kumari, Partam Manalai, Sharlene Leong, Alese Wooditch,William B Lawson and Malik Mansoor
Howard University, USAGeorge Mason University, USAThe University of Texas at Austin, USA
ScientificTracks Abstracts: J Addict Res Ther
Abstract
Background & Objectives: Opioid use disorders are common, chronic relapsing disorders. Buprenorphine (BUP) is an FDA approved medication in the treatment of opioid use disorders, but patient adherence to this medication remains a challenge. To identify risk factors for non-adherence, this chart review study examined the association between DSM-IV Axis I psychiatric disorders, substance use, demographics, and adherence to BUP-naloxone in African–American patients. Methods: Charts were selected of patients who had 5 visits and completed psychometric screens (patient health questionnaire, mood disorder questionnaire, and a posttraumatic stress disorder questionnaire) at the time of the initial visit (N ¼ 50). Urine drug screens (UDS) were also obtained. Treatment adherence was defined as BUP presence in UDS for 80% of the visits. Results: A total of 48% of patients were adherent to treatment. Non-adherent patients had higher rates of use for not only opioids, but also cocaine, and alcohol. Cocaine use was associated with BUP-naloxone non-adherence even after controlling for opioid use. Attendance in cognitive behavioral group therapy sessions (CBT) was significantly associated with adherence. Patients endorsing PTSD symptoms showed higher adherence to treatment compared to those who did not endorse these symptoms. Conclusions & Scientific Significance: Our results indicate that alcohol and illicit substance use is associated with nonadherence to BUP-naloxone treatment, and suggests that CBT and efforts to promote abstinence from non-opioid substance use may improve adherence among African–Americans. These findings contribute to growing literature on understanding adherence to BUP-naloxone, which is critical to reduce morbidity and mortality.Biography
Dr. Kumari received MD Degree from Dow Medical College Karachi, Pakistan. In addition, in 2004 she received a Master’s Degree in Public Health (MPH) from George Washington University, Washington DC. Dr. Kumari has a broad solid background in clinical psychiatry research, with specific training and expertise in managing, coordinating, and collaborating on multiple clinical research projects. As a project manager Dr. Kumari gained substantial research experience on NIDA funded research project- Seek Treat, Reach, and Identify pretrial Defendants Enhancement (STRIDE) in collaboration with the Yale School of Medicine, George Mason University and Howard University Hospital. The project STRIDE is a placebo-controlled, randomized controlled trial of buprenorphine treatment for HIV-infected,-infected, opioid dependent, community-supervised defendants or offenders.
E-mail: vsuneeta@yahoo.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi