Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
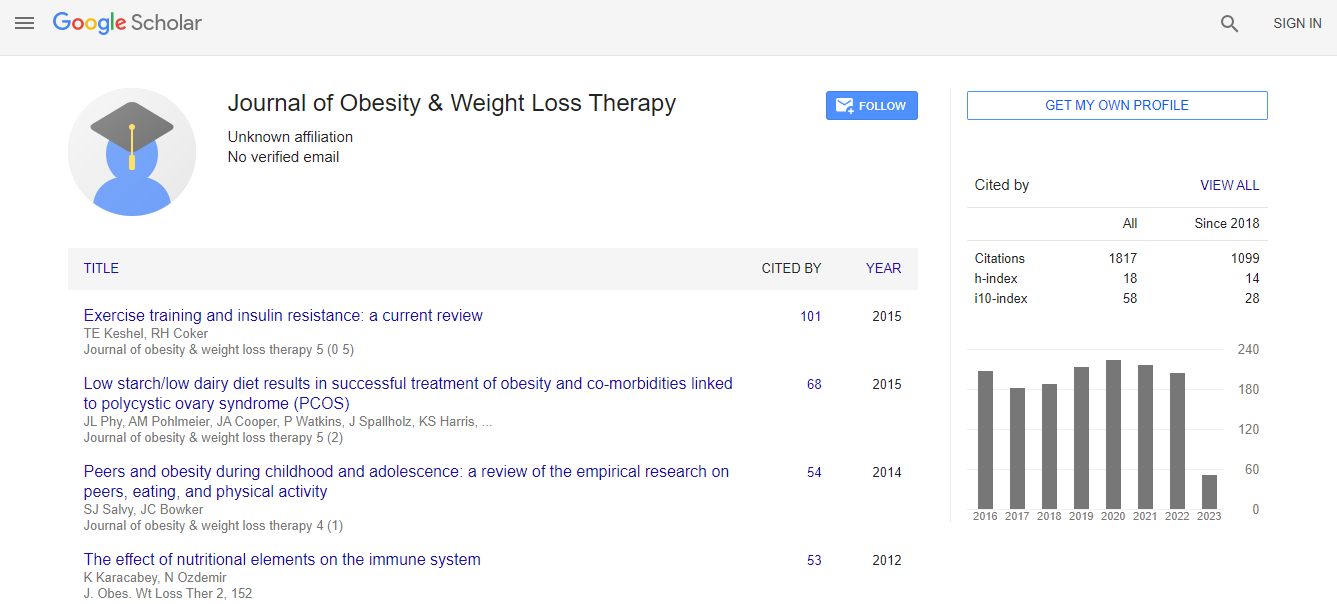
Google Scholar citation report
Citations : 2305
Journal of Obesity & Weight Loss Therapy received 2305 citations as per Google Scholar report
Journal of Obesity & Weight Loss Therapy peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- CABI full text
- Cab direct
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- University of Bristol
- Pubmed
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Arkray√ʬ?¬?s glucocard vital blood glucose monitoring system rates highly favourable in ease of use
3rd International Conference and Exhibition on Obesity & Weight Management
John Gleisner, Patricia Gill and Julie Walker
ARKRAY, USA
Posters: J Obes Weight Loss Ther
Abstract
Background: As part of the 510K approvals process for Blood Glucose Monitoring Systems (BGMS) in self-monitoring of Diabetes Mellitus, Ease of Use criterion are evaluated with questionnaires following participation in the clinical trial. It is critical that a BGMS be easy to use since it is an important tool in the management of diabetes. Without the ability to regulate one√ʬ?¬?s blood sugar, an individual is at risk for potential micro and macrovascular complications. Purpose: The objective of this study was to evaluate the Ease of Use as it relates to the ARKRAY GLUCOCARD Vital BGMS. Methods: A total of 118 subjects evaluated the ARKRAY GLUCOCARD Vital BGMS by answering a questionnaire directed at the Ease of Use of the device. Multiple topics were covered in the questionnaire including: Getting to know the meter system; Inserting a strip into the meter; Performing a blood glucose test; Reading meter display; Adding control solution to the test strip; Performing an AST test. The subjects were asked to rate the topics for the device as Very Easy, Easy, Ok, Difficult or Very Difficult. For evaluation purposes these topics were then grouped as Very Easy/Easy/Ok being considered positive responses and Difficult/Very Difficult as negative. Results: The first question addressing Getting to know the meter scored a 99.2% positive rating. Inserting a strip into the meter was also rated with a 99.2% positive rating. Performing a blood glucose test was rated a Very Easy/Easy/Ok by 100% of the participants. Reading meter display scored a 99.2% positive rating. Adding control solution to the test strip scored a 95.8% for being Very Easy/Easy/Ok to use. Performing an AST was rated as Very Easy/Easy/Ok by 93.2% of the subjects.Biography
John Gleisner completed his PhD in Biochemistry from the University of Minnesota and postdoctoral studies from the University of Iowa. His first career following graduate school was at the Virginia Mason Research Center in Seattle, WA. He later moved into industry where he is currently the Science Director at ARKRAY Factory USA in Minneapolis, MN. He has spent nearly 30 years working on blood glucose system development and support. He has authored over 25 publications and holds 11 US patents.
Email: walkerj@arkrayusa.com

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi