Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
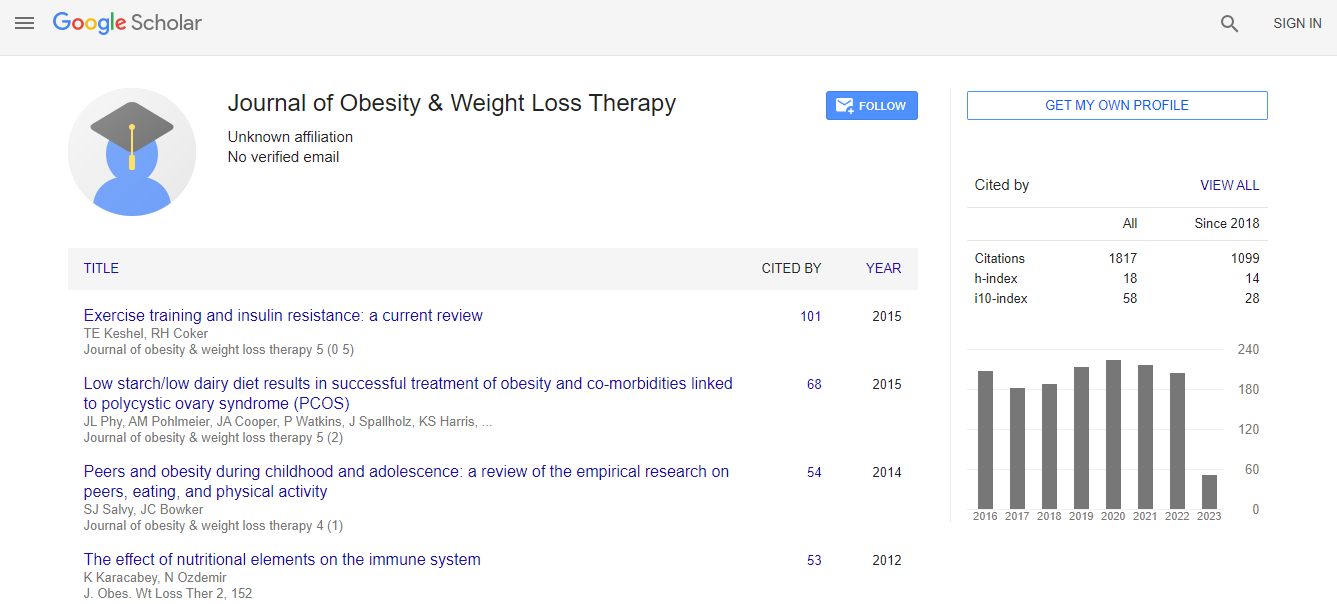
Google Scholar citation report
Citations : 2305
Journal of Obesity & Weight Loss Therapy received 2305 citations as per Google Scholar report
Journal of Obesity & Weight Loss Therapy peer review process verified at publons
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- CABI full text
- Cab direct
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- University of Bristol
- Pubmed
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Final results of multi-center, prospective, controlled trial of the duodenojejunal bypass liner for the treatment of type-2 diabetes mellitus in obese patients: Efficacy and factors predicting a suboptimal effect
JOINT EVENT 10th International Conference on Childhood Obesity and Nutrition & 2nd International Conference on Metabolic and Bariatric Surgery
Marek Benes, Spicak Julius, Drastich Pavel and Hucl Tomas
Institute of Clinical and Experimental Medicine, Czech Republic
ScientificTracks Abstracts: J Obes Weight Loss Ther
Abstract
Introduction: The global increase in obesity incidence results in an increase of type-2 diabetes mellitus (T2DM). Surgical treatment has proven to be effective; however, it carries a high risk of complications. The duodenal-jejunal bypass liner (EndoBarrier├?┬?├?┬«, GI Dynamics and EB) is an endoscopic implant that mimics the intestinal bypass portion of the Roux-en-Y gastric bypass. It results in weight loss and improvements in glucose control in obese patients with T2 diabetes mellitus (T2DM). Case Report: This is a final report of a prospective, controlled, multi-centre study aimed to determine the effectiveness of EB and to identify factors associated with a sub-optimal outcome of EB. Results: 70 subjects (45 with an implant, 25 controls) were included in the study. The groups were comparable with respect to age, gender, BMI (mean 41.7 vs. 39.5 kg/m2), T2DM duration (7.8 vs. 8.3 years), HbA1c level (88 vs. 86 mmol/mol) and T2DM treatment. In the EB group, all devices were successfully implanted. Only six devices had to be explanted prior to the end of the 10 months study period (bleeding, dislocation and need for ERCP because of choledocholithiasis). The mean procedure time was 17 minutes for an implantation and 16 minutes for an explanation. At 10 months, there was significantly greater weight loss and %EWL (19% vs. 7% and 43 vs. 12) and significantly improved long-term compensation of T2DM marker HbA1c (decreased by 25 vs. 10 mmol/mol) in the EB group. T2DM medicinal treatment could be reduced in more device subjects than controls. There was no serious adverse event. Mild abdominal pain and nausea after implantation were experienced by 60% of patients during first 14 days after implantation, 30% of patients during the first month and 10% of patients after one month. Lower initial BMI and lower body height were identified as negative prognostic factors for pain, but positive for efficacy of EB. Conclusion: The EB is safe when implanted for 10 months and results in significant weight loss and HbA1c reduction. This suggests that this novel device is a candidate for the primary therapy of morbid obesity and T2DM. Lower initial BMI and lower body height could be negative prognostic factor for pain, but positive for efficacy.Biography
Marek Benes completed his Graduation at 3rd Medical Faculty of Charles University in 2001. He was a Physician in Department of Gastroenterology and Hepatology at Institute of Clinical and Experimental Medicine in Prague. His main subjects of interest are Endoscopy and Bariatric Endoscopy.
Email: mabx@ikem.cz

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi